
Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act as autoreceptors in presynaptic histaminergic neurons and control histamine turnover by feedback inhibition of histamine synthesis and release. The H3 receptor has also been shown to presynaptically inhibit the release of a number of other neurotransmitters (i.e. it acts as an inhibitory heteroreceptor) including, but probably not limited to dopamine, GABA, acetylcholine, noradrenaline, histamine and serotonin.

ABT-239 is an H3-receptor inverse agonist developed by Abbott. It has stimulant and nootropic effects, and has been investigated as a treatment for ADHD, Alzheimer's disease, and schizophrenia. ABT-239 is more active at the human H3 receptor than comparable agents such as thioperamide, ciproxifan, and cipralisant. It was ultimately dropped from human trials after showing the dangerous cardiac side effect of QT prolongation, but is still widely used in animal research into H3 antagonists / inverse agonists.

Cipralisant (GT-2331, tentative trade name Perceptin) is an extremely potent histamine H3 receptor ligand originally developed by Gliatech. Cipralisant was initially classified as a selective H3 antagonist, but newer research (2005) suggests also agonist properties, i.e., functional selectivity.

Ciproxifan is an extremely potent histamine H3 inverse agonist/antagonist.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.

Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.
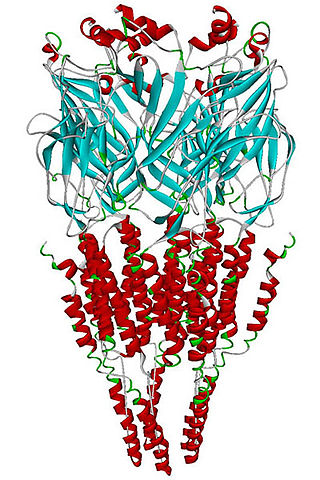
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].
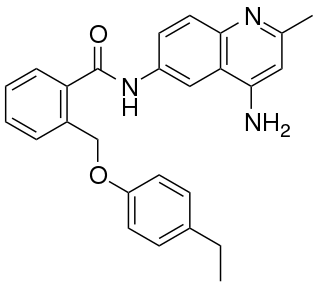
JTC-801 is an opioid analgesic drug used in scientific research.
An H3 receptor antagonist is a type of antihistaminic drug used to block the action of histamine at H3 receptors.

Lecozotan is an investigational drug by Wyeth tested for improvement of cognitive functions of Alzheimer's disease patients. As of June 2008, the first Phase III clinical trial has been completed.

SB-612,111 is an opioid receptor ligand which is a potent and selective antagonist for the nociceptin receptor (ORL-1), several times more potent than the older drug J-113,397. It does not have analgesic effects in its own right, but prevents the development of hyperalgesia, and also shows antidepressant effects in animal studies.

Indantadol is a drug which was formerly being investigated as an anticonvulsant and neuroprotective and is now under development for the treatment of neuropathic pain and chronic cough in Europe by Vernalis and Chiesi. It acts as a competitive, reversible, and non-selective monoamine oxidase inhibitor, and as a low affinity, non-competitive NMDA receptor antagonist. A pilot study of indantadol for chronic cough was initiated in October 2009 and in April 2010 it failed to achieve significant efficacy in neuropathic pain in phase IIb clinical trials.
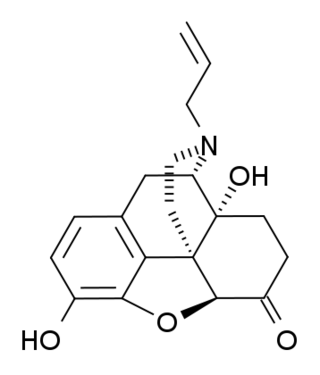
(+)-Naloxone (dextro-naloxone) is a drug which is the opposite enantiomer of the opioid antagonist drug (−)-naloxone. Unlike (-)-naloxone, (+)-naloxone has no significant affinity for opioid receptors, but instead has been discovered to act as a selective antagonist of Toll-like receptor 4. This receptor is involved in immune system responses, and activation of TLR4 induces glial activation and release of inflammatory mediators such as TNF-α and Interleukin-1.
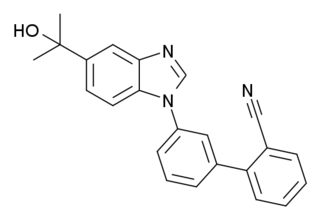
NS-11394 is a drug which acts as a subtype-selective positive allosteric modulator at GABAA receptors, with selectivity for the α3 and α5 subtypes. It has been researched as an analgesic for use in chronic or neuropathic pain.
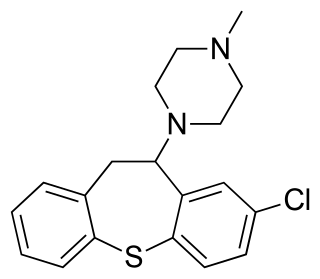
Clorotepine, also known as octoclothepin or octoclothepine, is an antipsychotic of the tricyclic group which was derived from perathiepin in 1965 and marketed in the Czech Republic by Spofa in or around 1971 for the treatment of schizophrenic psychosis.

AM-1714 (part of the AM cannabinoid series) is a drug that acts as a reasonably selective agonist of the peripheral cannabinoid receptor CB2, with sub-nanomolar affinity and 490x selectivity over the related CB1 receptor. In animal studies it has both analgesic and anti-allodynia effects. The 9-methoxy derivative AM-1710 has similar CB2 affinity but only 54x selectivity over CB1.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

JNJ-18038683 is a potent and selective antagonist of the 5HT7 serotonin receptor discovered by Johnson & Johnson. It has nootropic and antidepressant effects in both animal and human studies and has progressed to Phase II trials as an adjunctive treatment for improving cognition and mood in stable bipolar disorder; it has been found to reduce REM sleep (the lightest stage of sleep, elevated in depression) in humans and block circadian rhythm phase-shift advances in mice.
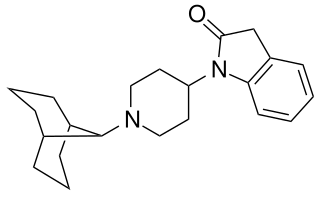
SR-16435 is a drug which acts as a potent partial agonist at both the μ-opioid receptor and nociceptin receptor. In animal studies it was found to be a potent analgesic, with results suggestive of reduced development of tolerance and increased activity against neuropathic pain compared to classic μ-selective agonists.


















