An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies.

Allergic rhinitis, of which the seasonal type is called hay fever, is a type of inflammation in the nose that occurs when the immune system overreacts to allergens in the air. Signs and symptoms include a runny or stuffy nose, sneezing, red, itchy, and watery eyes, and swelling around the eyes. The fluid from the nose is usually clear. Symptom onset is often within minutes following allergen exposure, and can affect sleep and the ability to work or study. Some people may develop symptoms only during specific times of the year, often as a result of pollen exposure. Many people with allergic rhinitis also have asthma, allergic conjunctivitis, or atopic dermatitis.

Rhinitis, also known as coryza, is irritation and inflammation of the mucous membrane inside the nose. Common symptoms are a stuffy nose, runny nose, sneezing, and post-nasal drip.

Cetirizine is a second-generation antihistamine used to treat allergic rhinitis, dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes and last for about a day. The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.
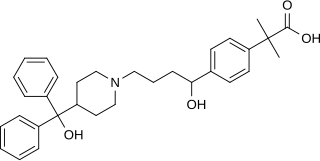
Fexofenadine, sold under the brand name Allegra among others, is an antihistamine pharmaceutical drug used in the treatment of allergy symptoms, such as hay fever and urticaria.

Nasal sprays are used to deliver medications locally in the nasal cavities or systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some situations, the nasal delivery route is preferred for systemic therapy because it provides an agreeable alternative to injection or pills. Substances can be assimilated extremely quickly and directly through the nose. Many pharmaceutical drugs exist as nasal sprays for systemic administration. Other applications include hormone replacement therapy, treatment of Alzheimer's disease and Parkinson's disease. Nasal sprays are seen as a more efficient way of transporting drugs with potential use in crossing the blood–brain barrier.

Phenylephrine is a medication used as a decongestant for uncomplicated nasal congestion, used to dilate the pupil, used to increase blood pressure, and used to relieve hemorrhoids. It can be taken by mouth, as a nasal spray, given by injection into a vein or muscle, or applied to the skin.

Allergen immunotherapy, also known as desensitization or hypo-sensitization, is a medical treatment for environmental allergies, such as insect bites, and asthma. Immunotherapy involves exposing people to larger and larger amounts of allergens in an attempt to change the immune system's response.

Olopatadine, sold under the brand name Patanol among others, is an antihistamine medication used to decrease the symptoms of allergic conjunctivitis and allergic rhinitis. It is used as eye drops or as a nasal spray. The eye drops generally result in an improvement within half an hour.
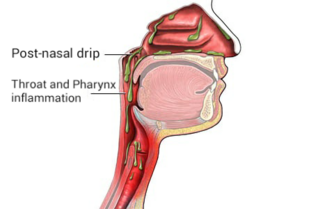
Post-nasal drip (PND), also known as upper airway cough syndrome (UACS), occurs when excessive mucus is produced by the nasal mucosa. The excess mucus accumulates in the back of the nose, and eventually in the throat once it drips down the back of the throat. It can be caused by rhinitis, sinusitis, gastroesophageal reflux disease (GERD), or by a disorder of swallowing. Other causes can be allergy, cold, flu, and side effects from medications.

Levocetirizine, sold under the brand name Xyzal, among others, is a second-generation antihistamine used for the treatment of allergic rhinitis and long-term hives of unclear cause. It is less sedating than older antihistamines. It is taken by mouth.

Ketotifen is an antihistamine medication and a mast cell stabilizer commonly used to treat allergic conditions such as conjunctivitis, asthma, and urticaria (hives). Ketotifen is available in ophthalmic and oral forms: the ophthalmic form relieves eye itchiness and irritation associated with seasonal allergies, while the oral form helps prevent systemic conditions such as asthma attacks and allergic reactions. In addition to treating allergies, ketotifen has shown efficacy in managing systemic mast cell diseases such as mastocytosis and mast cell activation syndrome (MCAS), which involve abnormal accumulation or activation of mast cells throughout the body. Ketotifen is also used for other allergic-type conditions like atopic dermatitis (eczema) and food allergies.

Flunisolide is a corticosteroid often prescribed as treatment for allergic rhinitis. Intranasal corticosteroids are the most effective medication for controlling symptoms.
Rhinitis medicamentosa is a condition of rebound nasal congestion suspected to be brought on by extended use of topical decongestants and certain oral medications that constrict blood vessels in the lining of the nose, although evidence has been contradictory.

Mometasone, also known as mometasone y 3 s, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose. Mometasone furoate, not mometasone, is used in medical products.

Mast cell stabilizers are medications used to prevent or control certain allergic disorders. They block mast cell degranulation, stabilizing the cell and thereby preventing the release of histamine and related mediators. One suspected pharmacodynamic mechanism is the blocking of IgE-regulated calcium channels. Without intracellular calcium, the histamine vesicles cannot fuse to the cell membrane and degranulate.

Ebastine is a H1 antihistamine with low potential for causing drowsiness.
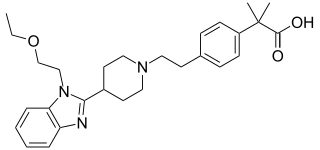
Bilastine is an antihistamine medication used to treat hives (urticaria), allergic rhinitis and itchy inflamed eyes (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.
Nonallergic rhinitis is rhinitis—inflammation of the inner part of the nose—not caused by an allergy. Nonallergic rhinitis displays symptoms including chronic sneezing or having a congested, drippy nose, without an identified allergic reaction. Other common terms for nonallergic rhinitis are vasomotor rhinitis and perennial rhinitis. The prevalence of nonallergic rhinitis in otolaryngology is 40%. Allergic rhinitis is more common than nonallergic rhinitis; however, both conditions have similar presentation, manifestation and treatment. Nasal itching and paroxysmal sneezing are usually associated with nonallergic rhinitis rather than allergic rhinitis.
Olopatadine/mometasone, sold under the brand name Ryaltris, is a fixed-dose combination medication for the treatment of allergic rhinitis and rhinoconjunctivitis in adults and adolescents twelve years of age and older. It contains olopatadine hydrochloride and mometasone furoate monohydrate. It is sprayed into the nose.


















