H1 antagonists, also called H1 blockers, are a class of medications that block the action of histamine at the H1 receptor, helping to relieve allergic reactions. Agents where the main therapeutic effect is mediated by negative modulation of histamine receptors are termed antihistamines; other agents may have antihistaminergic action but are not true antihistamines.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in combination with pseudoephedrine, a decongestant, known as loratadine/pseudoephedrine. It is taken orally.

Hydroxyzine, sold under the brand names Atarax and Vistaril among others, is an antihistamine medication. It is used in the treatment of itchiness, insomnia, anxiety, and nausea, including that due to motion sickness. It is used either by mouth or injection into a muscle.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Nortriptyline, sold under the brand name Pamelor, among others, is a medication used to treat depression. This medicine is also sometimes used for neuropathic pain, attention deficit hyperactivity disorder (ADHD), smoking cessation and anxiety. As with many antidepressants, its use for young people with depression and other psychiatric disorders may be limited due to increased suicidality in the 18–24 population initiating treatment. Nortriptyline is a less preferred treatment for ADHD and stopping smoking. It is taken by mouth.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.
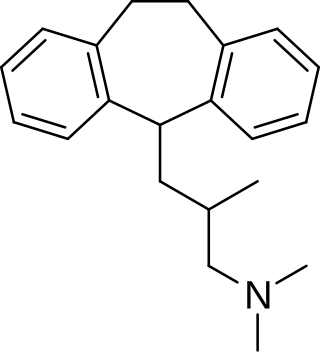
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.
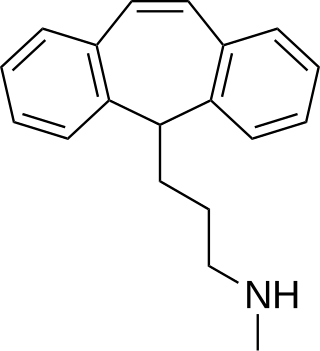
Protriptyline, sold under the brand name Vivactil among others, is a tricyclic antidepressant (TCA), specifically a secondary amine, indicated for the treatment of depression and attention-deficit hyperactivity disorder (ADHD). Uniquely among most of the TCAs, protriptyline tends to be energizing instead of sedating, and is sometimes used for narcolepsy to achieve a wakefulness-promoting effect.

Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia. It is taken by mouth.

Tricyclics are cyclic chemical compounds that contain three fused rings of atoms.

Cloperastine (INN) or cloperastin, in the forms of cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.

Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects. Antihistamines are usually for short-term treatment. Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection. Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Piperoxan, also known as benodaine, was the first antihistamine to be discovered. This compound, derived from benzodioxan, was prepared in the early 1930s by Daniel Bovet and Ernest Fourneau at the Pasteur Institute in France. Formerly investigated by Fourneau as an α-adrenergic-blocking agent, they demonstrated that it also antagonized histamine-induced bronchospasm in guinea pigs, and published their findings in 1933. Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution. One of Bovet and Fourneau's students, Anne-Marie Staub, published the first structure–activity relationship (SAR) study of antihistamines in 1939. Piperoxan and analogues themselves were not clinically useful due to the production of toxic effects in humans and were followed by phenbenzamine (Antergan) in the early 1940s, which was the first antihistamine to be marketed for medical use.
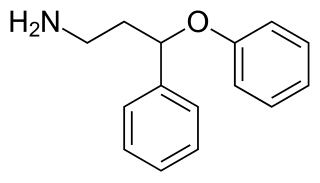
PPPA, or 3-phenoxy-3-phenylpropan-1-amine, is a drug which is described as an antidepressant. It was derived by Eli Lilly from the antihistamine diphenhydramine, a 2-diphenylmethoxyethanamine derivative with additional properties as a selective serotonin reuptake inhibitor (SSRI), and has been the basis for the subsequent discovery of a number of other antidepressant drugs.

Acylureas are a class of chemical compounds formally derived from the acylation of urea.

Fenethazine (INN), or phenethazine, is a first-generation antihistamine of the phenothiazine group. Promethazine and chlorpromazine, were derived from fenethazine. Fenethazine, in turn, was derived from phenbenzamine.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.
In pharmacology and pharmaceutics, a prototype drug is an individual drug that represents a drug class – group of medications having similar chemical structures, mechanism of action and mode of action. Prototypes are the most important, and typically the first developed drugs within the class, and are used as a reference to which all other drugs are compared.



















