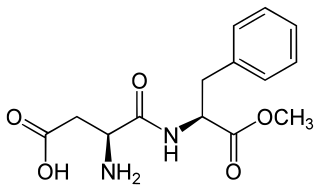
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Stevia is a sweet sugar substitute that is about 50 to 300 times sweeter than sugar. It is extracted from the leaves of Stevia rebaudiana, a plant native to areas of Paraguay and Brazil in the southern Amazon rainforest. The active compounds in stevia are steviol glycosides. Stevia is heat-stable, pH-stable, and not fermentable. Humans cannot metabolize the glycosides in stevia, and therefore it has zero calories. Its taste has a slower onset and longer duration than that of sugar, and at high concentrations some of its extracts may have an aftertaste described as licorice-like or bitter. Stevia is used in sugar- and calorie-reduced food and beverage products as an alternative for variants with sugar.

Splenda is a global brand of sugar substitutes and reduced-calorie food products. While the company is known for its original formulation containing sucralose, it also manufactures items using natural sweeteners such as stevia, monk fruit and allulose. It is owned by the American company Heartland Food Products Group. The high-intensity sweetener ingredient sucralose used in Splenda Original is manufactured by the British company Tate & Lyle.

A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

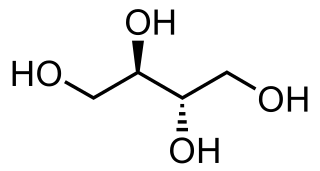
Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It is classified as a polyalcohol and a sugar alcohol, specifically an alditol. The name derives from Ancient Greek: ξύλον, xyl[on] 'wood', with the suffix -itol used to denote it being a sugar alcohol.

Saccharin, also called saccharine, benzosulfimide, or E954, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener. Saccharin is a benzoic sulfimide that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines. It appears as white crystals and is odorless.

Acesulfame potassium, also known as acesulfame K or Ace K, is a synthetic calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

Pepsi One, corporately styled PEPSI ONE, was a sugar-free cola, marketed by PepsiCo in the United States as an alternative to regular Pepsi and Diet Pepsi.

Diet or light beverages are generally sugar-free, artificially sweetened beverages with few or no calories. They are marketed for diabetics and other people who want to reduce their sugar and/or caloric intake.
Erythritol (, ) is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol). It is the reduced form of either D- or L-erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is C
4H
10O
4, or HO(CH2)(CHOH)2(CH2)OH.
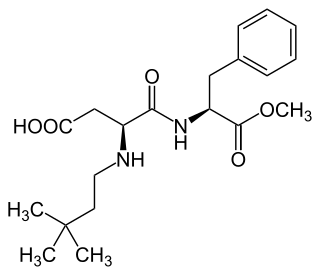
Neotame, also known by the trade name Newtame, is a non-caloric artificial sweetener and aspartame analog by NutraSweet. By mass, it is 8000 times sweeter than sucrose. It has no notable off-flavors when compared to sucrose. It enhances original food flavors. It can be used alone, but is often mixed with other sweeteners to increase their individual sweetness and decrease their off-flavors. It is chemically somewhat more stable than aspartame. Its use can be cost effective in comparison to other sweeteners as smaller amounts of neotame are needed.

Maltodextrin is a name shared by two different families of chemicals. Both families are glucose polymers, but have little chemical or nutritional similarity.

Diet Pepsi is a diet carbonated cola soft drink produced by PepsiCo, introduced in 1964 as a variant of Pepsi with no sugar. First test marketed in 1963 under the name Patio Diet Cola, it was re-branded as Diet Pepsi the following year, becoming the first diet cola to be distributed on a national scale in the United States. In the 1960s and 1970s, its competition consisted of the Coca-Cola Company's subsequently discontinued Tab. The United States represents the largest single market for Diet Pepsi.

Tagatose is a hexose monosaccharide. It is found in small quantities in a variety of foods, and has attracted attention as an alternative sweetener. It is often found in dairy products, because it is formed when milk is heated. It is similar in texture and appearance to sucrose :215 and is 92% as sweet,:198 but with only 38% of the calories.:209 Tagatose is generally recognized as safe by the Food and Agriculture Organization and the World Health Organization, and has been since 2001. Since it is metabolized differently from sucrose, tagatose has a minimal effect on blood glucose and insulin levels. Tagatose is also approved as a tooth-friendly ingredient for dental products. Consumption of more than about 30 grams of tagatose in a dose may cause gastric disturbance in some people, as it is mostly processed in the large intestine, similar to soluble fiber.:214

Equal is an American brand of artificial sweetener containing aspartame, acesulfame potassium, dextrose and maltodextrin. It is marketed as a tabletop sweetener by Merisant, a global corporation which also previously owned the well-known NutraSweet brand when it was a subsidiary of Monsanto and which has headquarters in Chicago, Illinois, Switzerland, Mexico, and Singapore. In French Canada, Equal is known as "Égal".

High-fructose corn syrup (HFCS), also known as glucose–fructose, isoglucose and glucose–fructose syrup, is a sweetener made from corn starch. As in the production of conventional corn syrup, the starch is broken down into glucose by enzymes. To make HFCS, the corn syrup is further processed by D-xylose isomerase to convert some of its glucose into fructose. HFCS was first marketed in the early 1970s by the Clinton Corn Processing Company, together with the Japanese Agency of Industrial Science and Technology, where the enzyme was discovered in 1965.

D-Psicose (C6H12O6), also known as D-allulose, or simply allulose, is a low-calorie epimer of the monosaccharide sugar fructose, used by some major commercial food and beverage manufacturers as a low-calorie sweetener. First identified in wheat in the 1940s, allulose is naturally present in small quantities in certain foods.
Fruit2O, formerly manufactured by Kraft, is a lightly flavored, non-carbonated water beverage introduced in 1999. Fruit2o was introduced to compete not only with the bottled water market but also with the soft drink market. Sunny Delight Beverages purchased the Veryfine Products line from Kraft in 2007.
Russell L. Blaylock is an author and a retired U.S. neurosurgeon.
Truvia is a brand of stevia-based sugar substitute developed jointly by The Coca-Cola Company and Cargill. It is distributed and marketed by Cargill as a tabletop sweetener as well as a food ingredient. Truvia is made of stevia leaf extract, erythritol, and natural flavors. Because it comes from the stevia plant, Cargill classifies Truvia as a natural sweetener in addition to being a non-nutritive sweetener, although Cargill has settled lawsuits alleging deceptive marketing of Truvia as "natural". Since its launch in 2008, Truvia natural sweetener has become the second best-selling sugar substitute in units in the U.S. behind Splenda, surpassing Equal and Sweet'n Low. Truvia competes with Stevia In The Raw, the #2 brand of stevia, owned by Cumberland Packaging who also makes Sweet 'n Low.