
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious.
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.

Malachite green is an organic compound that is used as a dyestuff and controversially as an antimicrobial in aquaculture. Malachite green is traditionally used as a dye for materials such as silk, leather, and paper. Despite its name the dye is not prepared from the mineral malachite; the name just comes from the similarity of color.
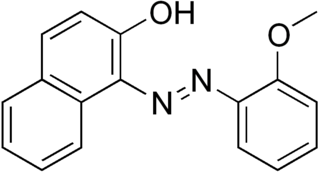
Sudan Red G is a yellowish red lysochrome azo dye. It has the appearance of an odorless reddish-orange powder with melting point 225 °C. It is soluble in fats and used for coloring of fats, oils, and waxes, including the waxes used in turpentine-based polishes. It is also used in polystyrene, cellulose, and synthetic lacquers. It is insoluble in water. It is stable to temperatures of about 100–110 °C. It was formerly used as a food dye, but still appears to be used for this purpose in china. It is used in some temporary tattoos, where it can cause contact dermatitis. It is also used in hair dyes. It is a component of some newer formulas for red smoke signals and smoke-screens, together with Disperse Red 11.

2-Naphthylamine is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.
4-Aminobiphenyl (4-ABP) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure. They are especially useful in quantifying an organism's exposure to a carcinogen. The presence of such an adduct indicates prior exposure to a potential carcinogen, but it does not necessarily indicate the presence of cancer in the subject animal.

o-Toluidine (ortho-toluidine) is an organic compound with the chemical formula CH3C6H4NH2. It is the most important of the three isomeric toluidines. It is a colorless liquid although commercial samples are often yellowish. It is a precursor to the herbicides metolachlor and acetochlor.

2-Acetylaminofluorene is a carcinogenic and mutagenic derivative of fluorene. It is used as a biochemical tool in the study of carcinogenesis. It induces tumors in a number of species in the liver, bladder and kidney. The metabolism of this compound in the body by means of biotransformation reactions is the key to its carcinogenicity. 2-AAF is a substrate for cytochrome P-450 (CYP) enzyme, which is a part of a super family found in almost all organisms. This reaction results in the formation of hydroxyacetylaminofluorene which is a proximal carcinogen and is more potent than the parent molecule. The N-hydroxy metabolite undergoes several enzymatic and non-enzymatic rearrangements. It can be O-acetylated by cytosolic N-acetyltransferase enzyme to yield N-acetyl-N-acetoxyaminofluorene. This intermediate can spontaneously rearrange to form the arylamidonium ion and a carbonium ion which can interact directly with DNA to produce DNA adducts. In addition to esterification by acetylation, the N-hydroxy derivative can be O-sulfated by cytosolic sulfur transferase enzyme giving rise to the N-acetyl-N-sulfoxy product.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.

Riddelliine is a chemical compound classified as a pyrrolizidine alkaloid. It was first isolated from Senecio riddellii and is also found in a variety of plants including Jacobaea vulgaris, Senecio vulgaris, and others plants in the genus Senecio.

PhIP (2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) is one of the most abundant heterocyclic amines (HCAs) in cooked meat. PhIP is formed at high temperatures from the reaction between creatine or creatinine, amino acids, and sugar. PhIP formation increases with the temperature and duration of cooking and also depends on the method of cooking and the variety of meat being cooked. The U.S. Department of Health and Human Services National Toxicology Program has declared PhIP as "reasonably anticipated to be a human carcinogen". International Agency for Research on Cancer (IARC), part of World Health Organization, has classified PhIP as IARC Group 2B carcinogen. There is sufficient evidence in experimental animals, as well as in vitro models, for the carcinogenicity of PhIP.
Toxicodynamics, termed pharmacodynamics in pharmacology, describes the dynamic interactions of a toxicant with a biological target and its biological effects. A biological target, also known as the site of action, can be binding proteins, ion channels, DNA, or a variety of other receptors. When a toxicant enters an organism, it can interact with these receptors and produce structural or functional alterations. The mechanism of action of the toxicant, as determined by a toxicant’s chemical properties, will determine what receptors are targeted and the overall toxic effect at the cellular level and organismal level.
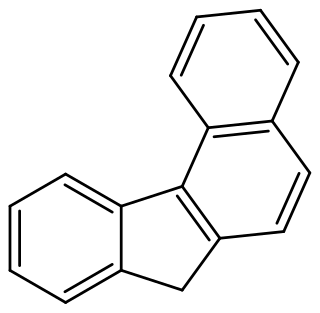
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.

Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule.

4-Ipomeanol (4-IPO) is a pulmonary pre-toxin isolated from sweet potatoes infected with the fungus Fusarium solani. One of the 4-IPO metabolites is toxic to the lungs, liver and kidney in humans and animals. This metabolite can covalently bind to proteins, thereby interfering with normal cell processes.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.

Elizabeth Cavert Miller was an American biochemist, known for fundamental research into the chemical mechanism of cancer carcinogenesis, working closely with her husband James A. Miller.




















