
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
SKF-82,958 is a synthetic compound of the benzazepine class that acts as a D1/D5 receptor full agonist. SKF-82,958 and similar D1-like-selective full agonists like SKF-81,297 and 6-Br-APB produce characteristic anorectic effects, hyperactivity and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine. SKF-82,958 was also subsequently found to act as an agonist of ERα with negligible activity at ERβ, making it a subtype-selective estrogen.
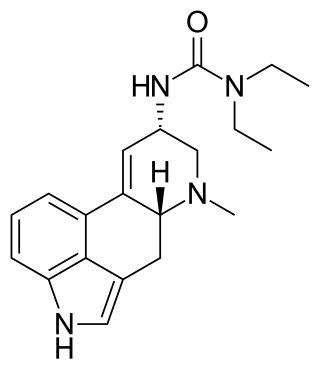
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.

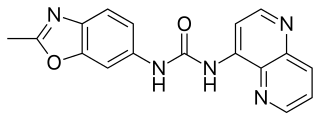
SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.
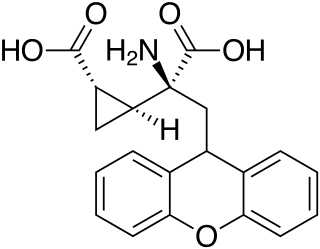
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

CP-94253 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 25x and 40x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It has a range of behavioral effects, based on animal testing. The effects include the following: promoting wakefulness by increasing dopamine release in the brain; reducing food intake and promoting satiety; enhancing the reinforcing effects of cocaine; and possible antidepressant effects.A recent study found that "Regardless of sex, CP94253 decreased cocaine intake after abstinence and during resumption of SA [self-administration] and decreased cue reactivity" suggesting that agonism of the inhibitory 5-HT2B receptors may diminish the cognitive reward of cocaine usage and increased use of the drug without a period of abstinence may be a product of test subjects trying to achieve a previously rewarding experience through larger dosages of cocaine.
SB-334867 is an orexin antagonist. It was the first non-peptide antagonist developed that is selective for the orexin receptor subtype OX1, with around 50x selectivity for OX1 over OX2 receptors. It has been shown to produce sedative and anorectic effects in animals, and has been useful in characterising the orexinergic regulation of brain systems involved with appetite and sleep, as well as other physiological processes. The hydrochloride salt of SB-334867 has been demonstrated to be hydrolytically unstable, both in solution and as the solid. Orexin antagonists have multiple potential clinical applications including the treatment of drug addiction, insomnia, obesity and diabetes.

UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. This causes dopamine release in the brain and has a stimulant effect, as well as blocking the behavioural effects of cocaine. It may also serve as a 5-HT2A receptor agonist, based on animal studies. It was investigated in clinical trials for the treatment of schizophrenia, but unexpectedly caused symptoms to become worse.
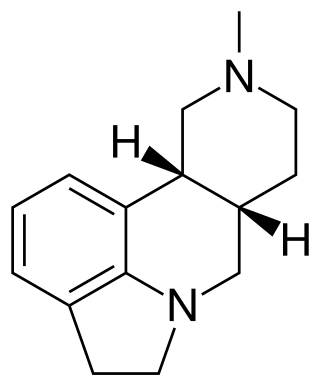
SDZ SER-082 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors, with good selectivity over other serotonin receptor subtypes and slight preference for 5-HT2C over 5-HT2B. It has been used in animal studies into the behavioural effects of the different 5-HT2 subtypes, and how they influence the effects of other drugs such as cocaine.
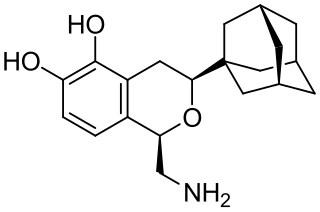
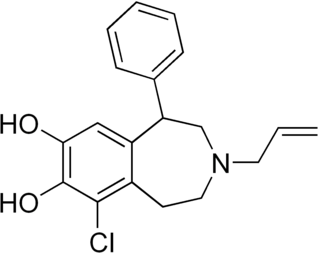
A-77636 is a synthetic drug which acts as a selective D1 receptor full agonist. It has nootropic, anorectic, rewarding and antiparkinsonian effects in animal studies, but its high potency and long duration of action causes D1 receptor downregulation and tachyphylaxis, and unlike other D1 full agonists such as SKF-82,958, it does not produce place preference in animals. A-77636 partially substituted for cocaine in animal studies, and has been suggested for use as a possible substitute drug in treating addiction, but it is better known for its use in studying the role of D1 receptors in the brain.

7-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with reasonable selectivity for the D3 receptor subtype, and low affinity for serotonin receptors, unlike its structural isomer 8-OH-DPAT. 7-OH-DPAT is self-administered in several animal models, and is used to study addiction to cocaine.
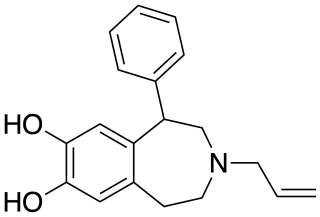
SKF-77,434 is a drug which acts as a selective dopamine D1 receptor partial agonist, and has stimulant and anorectic effects. Unlike other D1 agonists with higher efficacy such as SKF-81,297 and 6-Br-APB, SKF-77,434 does not maintain self-administration in animal studies, and so has been researched as a potential treatment for cocaine addiction.
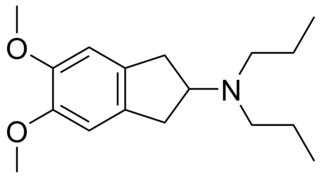
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.
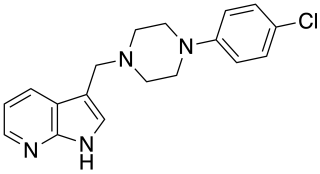
L-745,870 is a drug which acts as a dopamine receptor antagonist selective for the D4 subtype, and has antipsychotic effects in animal models, though it was not effective in human trials.

BP-897 is a drug used in scientific research which acts as a potent selective dopamine D3 receptor partial agonist with an in vitro intrinsic activity of ~0.6 and ~70x greater affinity for D3 over D2 receptors and is suspected to have partial agonist or antagonist activity in vivo. It has mainly been used in the study of treatments for cocaine addiction. A study comparing BP-897 with the potent, antagonistic, and highly D3 selective SB-277,011-A found, "SB 277011-A (1–10 mg/kg) was able to block cue-induced reinstatement of nicotine-seeking, indicating that DRD3 selective antagonism may be an effective approach to prevent relapse for nicotine. In contrast, BP 897 did not block the cue-induced reinstatement of nicotine-seeking or nicotine-taking under the FR5 schedule."

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.




















