
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin calx "lime", which was obtained from heating limestone.

Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.

Ultrastructure is the architecture of cells and biomaterials that is visible at higher magnifications than found on a standard optical light microscope. This traditionally meant the resolution and magnification range of a conventional transmission electron microscope (TEM) when viewing biological specimens such as cells, tissue, or organs. Ultrastructure can also be viewed with scanning electron microscopy and super-resolution microscopy, although TEM is a standard histology technique for viewing ultrastructure. Such cellular structures as organelles, which allow the cell to function properly within its specified environment, can be examined at the ultrastructural level.
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white solids of nutritional value and are found in many living organisms, e.g., bone mineral and tooth enamel. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals are used in the production of phosphoric acid and fertilizers. Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals).

Hydroxyapatite is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), often written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. It is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride or chloride, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis.

Bioglass 45S5 or calcium sodium phosphosilicate, is a bioactive glass specifically composed of 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, and 6.0 wt% P2O5. Typical applications of Bioglass 45S5 include: bone grafting biomaterials, repair of periodontal defects, cranial and maxillofacial repair, wound care, blood loss control, stimulation of vascular regeneration, and nerve repair.
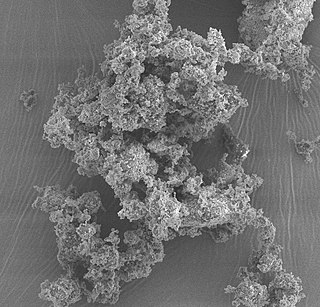
Bioactive glasses are a group of surface reactive glass-ceramic biomaterials and include the original bioactive glass, Bioglass. The biocompatibility and bioactivity of these glasses has led them to be used as implant devices in the human body to repair and replace diseased or damaged bones. Most bioactive glasses are silicate-based glasses that are degradable in body fluids and can act as a vehicle for delivering ions beneficial for healing. Bioactive glass is differentiated from other synthetic bone grafting biomaterials, in that it is the only one with anti-infective and angiogenic properties.
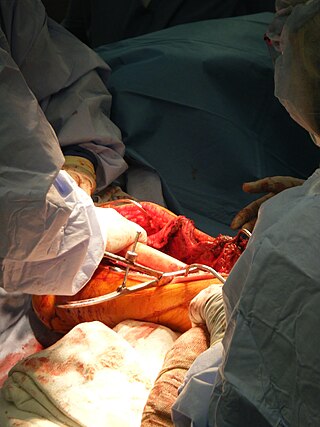
Bone grafting is a surgical procedure that replaces missing bone in order to repair bone fractures that are extremely complex, pose a significant health risk to the patient, or fail to heal properly. Some small or acute fractures can be cured without bone grafting, but the risk is greater for large fractures like compound fractures.

Calcium pyrophosphate (Ca2P2O7) is a chemical compound, an insoluble calcium salt containing the pyrophosphate anion. There are a number of forms reported: an anhydrous form, a dihydrate, Ca2P2O7·2H2O and a tetrahydrate, Ca2P2O7·4H2O. Deposition of dihydrate crystals in cartilage are responsible for the severe joint pain in cases of calcium pyrophosphate deposition disease (pseudo gout) whose symptoms are similar to those of gout. Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes, because of its insolubility and nonreactivity toward fluoride.

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.
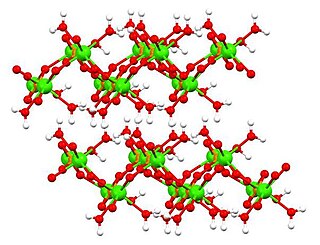
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Phosphorylase kinase (PhK) is a serine/threonine-specific protein kinase which activates glycogen phosphorylase to release glucose-1-phosphate from glycogen. PhK phosphorylates glycogen phosphorylase at two serine residues, triggering a conformational shift which favors the more active glycogen phosphorylase “a” form over the less active glycogen phosphorylase b.
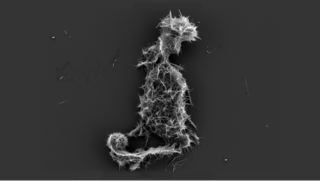
Amorphous calcium phosphate (ACP) is a glassy solid that is formed from the chemical decomposition of a mixture of dissolved phosphate and calcium salts (e.g. (NH4)2HPO4 + Ca(NO3)2). The resulting amorphous mixture consists mostly of calcium and phosphate, but also contains varying amounts of water and hydrogen and hydroxide ions, depending on the synthesis conditions. Such mixtures are also known as calcium phosphate cement.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.
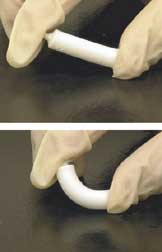
Artificial bone refers to bone-like material created in a laboratory that can be used in bone grafts, to replace human bone that was lost due to severe fractures, disease, etc.
Octacalcium phosphate (sometimes referred to as OCP) is a form of calcium phosphate with formula Ca8H2(PO4)6·5H2O. OCP may be a precursor to tooth enamel, dentine, and bones. OCP is a precursor of hydroxyapatite (HA), an inorganic biomineral that is important in bone growth. OCP has garnered lots of attention due to its inherent biocompatibility. While OCP exhibits good properties in terms of bone growth, very stringent synthesis requirements make it difficult for mass productions, but nevertheless has shown promise not only in-vitro, but also in in-vivo clinical case studies.
A simulated body fluid (SBF) is a solution with an ion concentration close to that of human blood plasma, kept under mild conditions of pH and identical physiological temperature. SBF was first introduced by Kokubo et al. in order to evaluate the changes on a surface of a bioactive glass ceramic. Later, cell culture media, in combination with some methodologies adopted in cell culture, were proposed as an alternative to conventional SBF in assessing the bioactivity of materials.

Chromium(III) phosphate describes inorganic compounds with the chemical formula CrPO4·(H2O)n, where n = 0, 4, or 6. All are deeply colored solids. Anhydrous CrPO4 is green. The hexahydrate CrPO4·6H2O is violet.
Tetracalcium phosphate is the compound Ca4(PO4)2O, (4CaO·P2O5). It is the most basic of the calcium phosphates, and has a Ca/P ratio of 2, making it the most phosphorus poor phosphate. It is found as the mineral hilgenstockite, which is formed in industrial phosphate rich slag (called "Thomas slag"). This slag was used as a fertiliser due to the higher solubility of tetracalcium phosphate relative to apatite minerals. Tetracalcium phosphate is a component in some calcium phosphate cements that have medical applications.
Vanadium phosphates are inorganic compounds with the formula VOxPO4 as well related hydrates with the formula VOxPO4(H2O)n. Some of these compounds are used commercially as catalysts for oxidation reactions.

















