
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
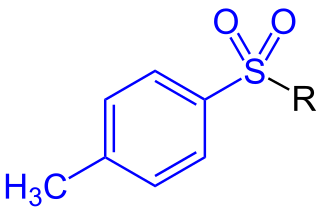
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula −SO2−C6H4−CH3. It consists of a tolyl group, −C6H4−CH3, joined to a sulfonyl group, −SO2−, with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, CH3C6H4SO2Cl (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, CH3C6H4SO2OH (abbreviated TsOH). The para orientation illustrated (p-toluenesulfonyl) is most common, and by convention tosyl without a prefix refers to the p-toluenesulfonyl group.

In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R−S(=O)2−O−, where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid. In salts, the mesylate is present as the CH3SO−3 anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group or anion, the spelling used is sometimes mesilate.

The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using carbon tetraiodide, methyl iodide or iodine gives alkyl iodides. The reaction is credited to and named after Rolf Appel, it had however been described earlier. The use of this reaction is becoming less common, due to carbon tetrachloride being restricted under the Montreal protocol.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Oxalyl chloride is an organic chemical compound with the formula Cl−C(=O)−C(=O)−Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.

p-Toluenesulfonic acid (PTSA, pTSA, or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.
Methanesulfonyl chloride is an organosulfur compound with the formula CH3SO2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl group CH3SO2–, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene.

Methanesulfonic acid (MsOH) or methanesulphonic acid is an organosulfuric, colorless liquid with the molecular formula CH3SO3H and structure H3C−S(=O)2−OH. It is the simplest of the alkylsulfonic acids. Salts and esters of methanesulfonic acid are known as mesylates. It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid.

Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.

Reductions with samarium(II) iodide involve the conversion of various classes of organic compounds into reduced products through the action of samarium(II) iodide, a mild one-electron reducing agent.



















