
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier in 1895. Most carboxylic acids are suitable for the reaction, but the alcohol should generally be primary or secondary. Tertiary alcohols are prone to elimination. Contrary to common misconception found in organic chemistry textbooks, phenols can also be esterified to give good to near quantitative yield of products. Commonly used catalysts for a Fischer esterification include sulfuric acid, p-toluenesulfonic acid, and Lewis acids such as scandium(III) triflate. For more valuable or sensitive substrates other, milder procedures such as Steglich esterification are used. The reaction is often carried out without a solvent or in a non-polar solvent to facilitate the Dean-Stark method. Typical reaction times vary from 1–10 hours at temperatures of 60-110 °C.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed acetate esters or simply acetates. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Oxalyl chloride is an organic chemical compound with the formula Cl−C(=O)−C(=O)−Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
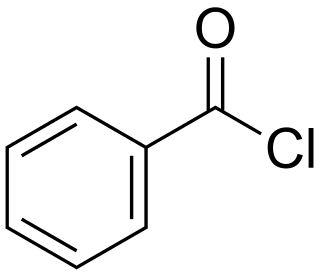
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C7H5ClO. It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring with an acyl chloride substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
Nucleophilic acyl substitution describes a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.
The Hunsdiecker reaction is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material and a halogen atom is introduced its place. A catalytic approach has been developed.
In organic chemistry, the acetoxy group, is a functional group with the formula −OCOCH3 and the structure −O−C(=O)−CH3. As the -oxy suffix implies, it differs from the acetyl group by the presence of an additional oxygen atom. The name acetoxy is the short form of acetyl-oxy.
Formyl fluoride is the organic compound with the formula HC(O)F.

Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidines.
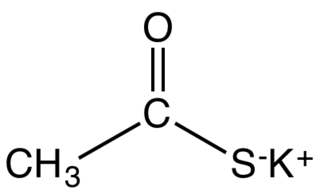
Potassium thioacetate is an organosulfur compound and a salt with the formula CH3COS−K+. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives.




















