
Erbium is a chemical element; it has symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, originally found in the gadolinite mine in Ytterby, Sweden, which is the source of the element's name.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.

Erbium(III) chloride is a violet solid with the formula ErCl3. It is used in the preparation of erbium metal.

Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form.
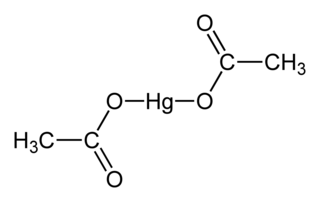
Mercury(II) acetate, also known as mercuric acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.

Erbium(III) oxide is the inorganic compound with the formula Er2O3. It is a pink paramagnetic solid. It finds uses in various optical materials.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Erbium(III) fluoride is the fluoride of erbium, a rare earth metal, with the chemical formula ErF3. It can be used to make infrared light-transmitting materials and up-converting luminescent materials.
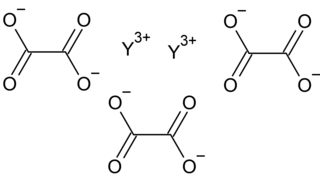
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

Erbium(III) nitrate is an inorganic compound, a salt of erbium and nitric acid with the chemical formula Er(NO3)3. The compound forms pink crystals, readily soluble in water, also forms crystalline hydrates.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Ytterbium(III) acetate is an inorganic salt of ytterbium and acetic acid, with a chemical formula of Yb(CH3COO)3. It has colorless crystals that are soluble in water and can form hydrates.

Lutetium(III) acetate is the acetate salt of lutetium with the chemical formula of Lu(CH3COO)3.

Dysprosium acetate is a hypothetical salt of dysprosium and acetate. Its proposed chemical formula is Dy(CH3COO)3.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3.

Thulium(III) acetate is the acetate salt of thulium, with the chemical formula of Tm(CH3COO)3. It can exist in the tetrahydrate or the anhydrous form.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.

Lanthanum acetylacetonate refers to the coordination complex with the formula La(C5H7O2)3. This anhydrous acetylacetonate complex has not been characterized well, but the dihydrate La(C5H7O2)3(H2O)2 has been characterized by X-ray crystallography.
Erbium nitride is a binary inorganic compound of erbium and nitrogen with the chemical formula ErN.


















