
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Lethal injection is the practice of injecting one or more drugs into a person for the express purpose of causing rapid death. The main application for this procedure is capital punishment, but the term may also be applied in a broader sense to include euthanasia and other forms of suicide. The drugs cause the person to become unconscious, stops their breathing, and causes a heart arrhythmia, in that order.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
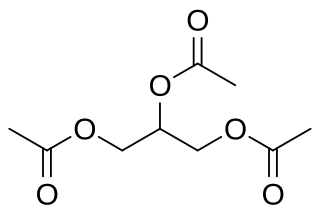
Triacetin is the organic compound with the formula C3H5(OCOCH3)3. It is classified as a triglyceride, i.e., the triester of glycerol with acetic acid. It is a colorless, viscous, and odorless liquid with a high boiling point and a low melting point. It has a mild, sweet taste in concentrations lower than 500 ppm, but may appear bitter at higher concentrations. It is one of the glycerine acetate compounds.
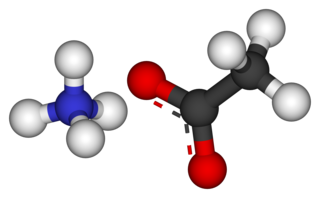
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially.
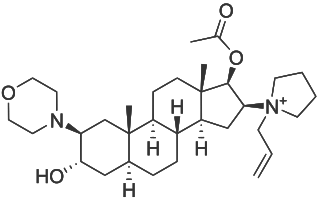
Rocuronium bromide is an aminosteroid non-depolarizing neuromuscular blocker or muscle relaxant used in modern anaesthesia to facilitate tracheal intubation by providing skeletal muscle relaxation, most commonly required for surgery or mechanical ventilation. It is used for standard endotracheal intubation, as well as for rapid sequence induction (RSI).

Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.
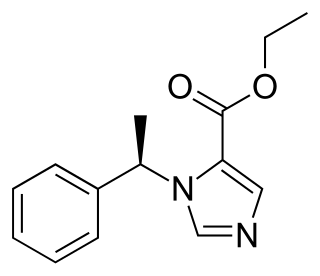
Etomidate is a short-acting intravenous anaesthetic agent used for the induction of general anaesthesia and sedation for short procedures such as reduction of dislocated joints, tracheal intubation, cardioversion and electroconvulsive therapy. It was developed at Janssen Pharmaceutica in 1964 and was introduced as an intravenous agent in 1972 in Europe and in 1983 in the United States.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.

Dipotassium phosphate (K2HPO4) (also dipotassium hydrogen orthophosphate; potassium phosphate dibasic) is the inorganic compound with the formula K2HPO4.(H2O)x (x = 0, 3, 6). Together with monopotassium phosphate (KH2PO4.(H2O)x), it is often used as a fertilizer, food additive, and buffering agent. It is a white or colorless solid that is soluble in water.
Calcium magnesium acetate (CMA) is a deicer and can be used as an alternative to road salt. It is approximately as corrosive as normal tap water, and in varying concentrations can be effective in stopping road ice from forming down to around −27.5 °C (−17.5 °F) (its eutectic temperature). CMA can also be used as an H2S capture agent.
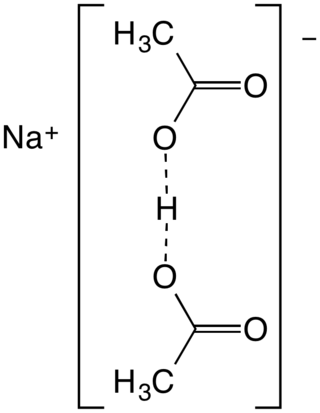
Sodium diacetate is a compound with formula NaH(C
2H
3O
2)
2. It is a salt of acetic acid. It is a colorless solid that is used in seasonings and as an antimicrobial agent.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Sodium salts are salts composed of a sodium cation and the conjugate base anion of some inorganic or organic acids. They can be formed by the neutralization of such acids with sodium hydroxide.

The execution of John Grant took place in the U.S. state of Oklahoma by means of lethal injection. Grant was sentenced to death for the 1998 murder of prison cafeteria worker Gay Carter.

Mark James Asay was an American spree killer who was executed by the state of Florida for the 1987 racially motivated murders of two men in Jacksonville, Florida. He was convicted, sentenced to death, and subsequently executed in 2017 at Florida State Prison by lethal injection. Asay's execution generated attention as it was noted by multiple news agencies that he was the first white person to be executed in Florida for killing a black person. He was also the first person to be executed in the United States using the drug etomidate.

Glossip v. Chandler is a United States District Court for the Western District of Oklahoma case in which the plaintiffs challenged the State of Oklahoma's execution protocol. The initial lawsuit, Glossip v. Gross, rose to the United States Supreme Court in 2015 at the preliminary injunction stage and involved an earlier version of Oklahoma's lethal injection protocol. The case was reopened in the District Court in 2020 following an end to Oklahoma's moratorium on executions.



















