
Chromium is a chemical element; it has symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).
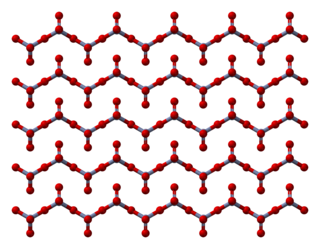
Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Potassium chromate is the inorganic compound with the formula K2CrO4. This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.

Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

Potassium chlorochromate is an inorganic compound with the formula KCrO3Cl. It is the potassium salt of chlorochromate, [CrO3Cl]−. It is a water-soluble orange compound is used occasionally for oxidation of organic compounds. It is sometimes called Péligot's salt, in recognition of its discoverer Eugène-Melchior Péligot.

Nickel(II) chromate (NiCrO4) is an acid-soluble compound, red-brown in color, with high tolerances for heat. It and the ions that compose it have been linked to tumor formation and gene mutation, particularly to wildlife.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores.

Potassium peroxochromate, potassium tetraperoxochromate(V), or simply potassium perchromate, is an inorganic compound having the chemical formula K3[Cr(O2)4]. It is a red-brown paramagnetic solid. It is the potassium salt of tetraperoxochromate(V), one of the few examples of chromium in the +5 oxidation state and one of the rare examples of a complex stabilized only by peroxide ligands. This compound is used as a source of singlet oxygen.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
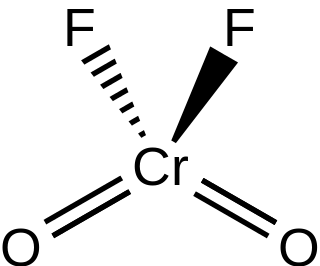
Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
Europium(III) chromate is a chemical compound composed of europium, chromium and oxygen with europium in the +3 oxidation state, chromium in the +5 oxidation state and oxygen in the −2 oxidation state. It has the chemical formula of EuCrO4.

















