An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.

Hair coloring, or hair dyeing, is the practice of changing the color of the hair on humans' heads. The main reasons for this are cosmetic: to cover gray or white hair, to alter hair to create a specific look, to change a color to suit preference or to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.

Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.
Lead(II) hydroxide, Pb(OH)2, is a hydroxide of lead, with lead in oxidation state +2.
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen (O).
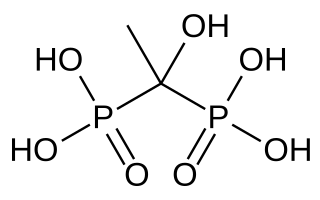
Etidronic acid, also known as etidronate, is a non-nitrogenous bisphosphonate used as a medication, detergent, water treatment, and cosmetic.
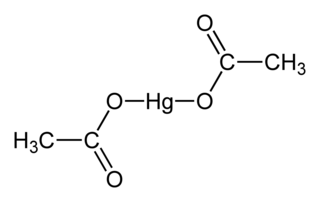
Mercury(II) acetate, also known as mercuric acetate is a chemical compound, the mercury(II) salt of acetic acid, with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.

Organolead chemistry is the scientific study of the synthesis and properties of organolead compounds, which are organometallic compounds containing a chemical bond between carbon and lead. The first organolead compound was hexaethyldilead (Pb2(C2H5)6), first synthesized in 1858. Sharing the same group with carbon, lead is tetravalent.
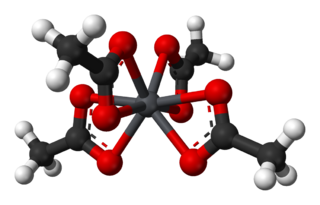
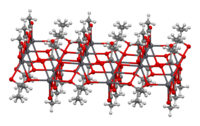
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.

Ferric acetate is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly the salt is known as "basic iron acetate". The formation of the red-brown complex was once used as a test for ferric ions.

Venetian ceruse or Venetian white, also known as blanc de céruse de Venise and Spirits of Saturn, was a 16th-century cosmetic used as a skin whitener. It was in great demand and considered the best available at the time, supposedly containing the best quality white lead sourced from Venice, the global merchant capital at the time. It is similar to the regular ceruse, although it was marketed as better, more exclusive and more expensive than the regular ceruse variant. The regular ceruse white pigment is a basic lead carbonate of chemical formula 2 PbCO
3·Pb(OH)
2 while the mineral cerussite is a simple carbonate of lead.

White lead is the basic lead carbonate 2PbCO3·Pb(OH)2. It is a complex salt, containing both carbonate and hydroxide ions. White lead occurs naturally as a mineral, in which context it is known as hydrocerussite, a hydrate of cerussite. It was formerly used as an ingredient for lead paint and a cosmetic called Venetian ceruse, because of its opacity and the satiny smooth mixture it made with dryable oils. However, it tended to cause lead poisoning, and its use has been banned in most countries.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.
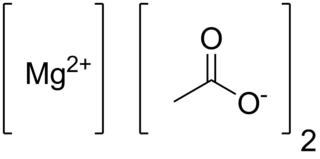
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
2-Ethoxyethyl acetate is an organic compound with the formula CH3CH2OCH2CH2O2CCH3. It is the ester of ethoxyethanol and acetic acid. A colorless liquid, it is partially soluble in water.
Lead stearate is a metal-organic compound, a salt of lead and stearic acid with the chemical formula C
36H
70PbO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid. The compound is toxic.



 [2]
[2]