
Migraine is a common neurological disorder characterized by recurrent headaches. Typically, the associated headache affects one side of the head, is pulsating in nature, may be moderate to severe in intensity, and could last from a few hours to three days. Non-headache symptoms may include nausea, vomiting, and sensitivity to light, sound, or smell. The pain is generally made worse by physical activity during an attack, although regular physical exercise may prevent future attacks. Up to one-third of people affected have aura: typically, it is a short period of visual disturbance that signals that the headache will soon occur. Occasionally, aura can occur with little or no headache following, but not everyone has this symptom.
Cluster headache (CH) is a neurological disorder characterized by recurrent severe headaches on one side of the head, typically around the eye(s). There is often accompanying eye watering, nasal congestion, or swelling around the eye on the affected side. These symptoms typically last 15 minutes to 3 hours. Attacks often occur in clusters which typically last for weeks or months and occasionally more than a year.

Transcutaneous electrical nerve stimulation is the use of electric current produced by a device to stimulate the nerves for therapeutic purposes. TENS, by definition, covers the complete range of transcutaneously applied currents used for nerve excitation although the term is often used with a more restrictive intent, namely to describe the kind of pulses produced by portable stimulators used to reduce pain. The unit is usually connected to the skin using two or more electrodes which are typically conductive gel pads. A typical battery-operated TENS unit is able to modulate pulse width, frequency, and intensity. Generally, TENS is applied at high frequency (>50 Hz) with an intensity below motor contraction or low frequency (<10 Hz) with an intensity that produces motor contraction. More recently, many TENS units use a mixed frequency mode which alleviates tolerance to repeated use. Intensity of stimulation should be strong but comfortable with greater intensities, regardless of frequency, producing the greatest analgesia. While the use of TENS has proved effective in clinical studies, there is controversy over which conditions the device should be used to treat.
Electroacupuncture is a form of acupuncture where a small electric current is passed between pairs of acupuncture needles. According to some acupuncturists, this practice augments the use of regular acupuncture, can restore health and well-being, and is particularly good for treating pain. There is evidence for some efficacy in treating moderate post-chemotherapy vomiting, but not for acute vomiting or delayed nausea severity.
Occipital neuralgia (ON) is a painful condition affecting the posterior head in the distributions of the greater occipital nerve (GON), lesser occipital nerve (LON), third occipital nerve (TON), or a combination of the three. It is paroxysmal, lasting from seconds to minutes, and often consists of lancinating pain that directly results from the pathology of one of these nerves. It is paramount that physicians understand the differential diagnosis for this condition and specific diagnostic criteria. There are multiple treatment modalities, several of which have well-established efficacy in treating this condition.

The pterygopalatine ganglion is a parasympathetic ganglion found in the pterygopalatine fossa. It is largely innervated by the greater petrosal nerve ; and its postsinaptic axons project to the lacrimal glands and nasal mucosa. The flow of blood to the nasal mucosa, in particular the venous plexus of the conchae, is regulated by the pterygopalatine ganglion and heats or cools the air in the nose. It is one of four parasympathetic ganglia of the head and neck, the others being the submandibular ganglion, otic ganglion, and ciliary ganglion.

Vagus nerve stimulation (VNS) is a medical treatment that involves delivering electrical impulses to the vagus nerve. It is used as an add-on treatment for certain types of intractable epilepsy and treatment-resistant depression.

A spinal cord stimulator (SCS) or dorsal column stimulator (DCS) is a type of implantable neuromodulation device that is used to send electrical signals to select areas of the spinal cord for the treatment of certain pain conditions. SCS is a consideration for people who have a pain condition that has not responded to more conservative therapy. There are also spinal cord stimulators under research and development that could enable patients with spinal cord injury to walk again via epidural electrical stimulation (EES).
Hemicrania continua (HC) is a persistent unilateral headache that responds to indomethacin. It is usually unremitting, but rare cases of remission have been documented. Hemicrania continua is considered a primary headache disorder, meaning that it is not caused by another condition.
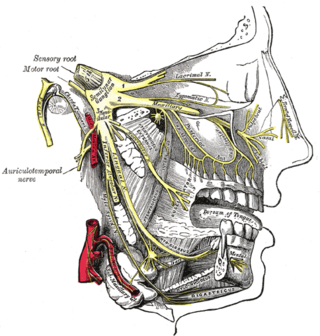
Migraine surgery is a surgical operation undertaken with the goal of reducing or preventing migraines. Migraine surgery most often refers to surgical decompression of one or several nerves in the head and neck which have been shown to trigger migraine symptoms in many migraine sufferers. Following the development of nerve decompression techniques for the relief of migraine pain in the year 2000, these procedures have been extensively studied and shown to be effective in appropriate candidates. The nerves that are most often addressed in migraine surgery are found outside of the skull, in the face and neck, and include the supra-orbital and supra-trochlear nerves in the forehead, the zygomaticotemporal nerve and auriculotemporal nerves in the temple region, and the greater occipital, lesser occipital, and third occipital nerves in the back of the neck. Nerve impingement in the nasal cavity has additionally been shown to be a trigger of migraine symptoms.
Electroanalgesia is a form of analgesia, or pain relief, that uses electricity to ease pain. Electrical devices can be internal or external, at the site of pain (local) or delocalized throughout the whole body. It works by interfering with the electric currents of pain signals, inhibiting them from reaching the brain and inducing a response; different from traditional analgesics, such as opiates which mimic natural endorphins and NSAIDs that help relieve inflammation and stop pain at the source. Electroanalgesia has a lower addictive potential and poses less health threats to the general public, but can cause serious health problems, even death, in people with other electrical devices such as pacemakers or internal hearing aids, or with heart problems.
Eugene G. Lipov, M.D. is a physician researcher and board-certified anesthesiologist who specializes in intervention-based pain management in the Chicago area.
Preventive treatment of migraine can be an important component of migraine management. Such treatments can take many forms, including everything from surgery, taking certain drugs or nutritional supplements, to lifestyle alterations such as increased exercise and avoidance of migraine triggers.
Multifocal motor neuropathy (MMN) is a progressively worsening condition where muscles in the extremities gradually weaken. The disorder, a pure motor neuropathy syndrome, is sometimes mistaken for amyotrophic lateral sclerosis (ALS) because of the similarity in the clinical picture, especially if muscle fasciculations are present. MMN is thought to be autoimmune. It was first described in the mid-1980s.
Calcitonin gene-related peptide (CGRP) receptor antagonists are a class of drugs that act as antagonists of the calcitonin gene-related peptide receptor (CGRPR).
Migraine treatment may be either prophylactic (preventive) or abortive (rescue). Prevention is better than cure, so the ideal treatment goal is to prevent migraine attacks. Because migraine is an exceedingly complex condition, there are various preventive treatments which have their effect by disrupting different links in the chain of events that occur during a migraine attack. As rescue treatments also target and disrupt different processes occurring during migraine, these are summarized, with their relative merits and demerits.
Neuromodulation is "the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body". It is carried out to normalize – or modulate – nervous tissue function. Neuromodulation is an evolving therapy that can involve a range of electromagnetic stimuli such as a magnetic field (rTMS), an electric current, or a drug instilled directly in the subdural space. Emerging applications involve targeted introduction of genes or gene regulators and light (optogenetics), and by 2014, these had been at minimum demonstrated in mammalian models, or first-in-human data had been acquired. The most clinical experience has been with electrical stimulation.
A peripheral nerve interface is the bridge between the peripheral nervous system and a computer interface which serves as a bi‐directional information transducer recording and sending signals between the human body and a machine processor. Interfaces to the nervous system usually take the form of electrodes for stimulation and recording, though chemical stimulation and sensing are possible. Research in this area is focused on developing peripheral nerve interfaces for the restoration of function following disease or injury to minimize associated losses. Peripheral nerve interfaces also enable electrical stimulation and recording of the peripheral nervous system to study the form and function of the peripheral nervous system. For example, recent animal studies have demonstrated high accuracy in tracking physiological meaningful measures, like joint angle. Many researchers also focus in the area of neuroprosthesis, linking the human nervous system to bionics in order to mimic natural sensorimotor control and function. Successful implantation of peripheral nerve interfaces depend on a number of factors which include appropriate indication, perioperative testing, differentiated planning, and functional training. Typically microelectrode devices are implanted adjacent to, around or within the nerve trunk to establish contact with the peripheral nervous system. Different approaches may be used depending on the type of signal desired and attainable.
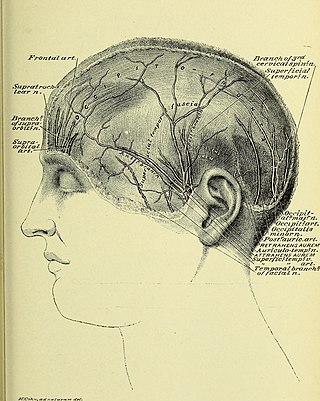
Occipital nerve block is a procedure involving injection of steroids or anesthetics into regions of the greater occipital nerve and the lesser occipital nerve used to treat chronic headaches.

Konstantin Slavin is a Professor and Head of the Department of Stereotactic and functional neurosurgery at the University of Illinois College of Medicine. He is a former president of the American Society for Stereotactic and functional neurosurgery and current vice-president of the World Society for Stereotactic and Functional Neurosurgery. His specialties include Aneurysm, Brain surgery, Brain Tumor, Cerebrovascular Disorders, Craniotomy, Dystonia, Essential Tremor, Facial Nerve Pain, Facial Pain, Glioblastoma, Headache disorders, Laminectomy, Lower back pain, Movement Disorders, Multiple Sclerosis, Neck Pain, Neurosurgery, Neurosurgical Procedures, Pain, Parkinson Disease, Spinal Cord Injuries, and Stroke.







