Related Research Articles

Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal with a density of 22.56 g/cm3 (0.815 lb/cu in) as defined by experimental X-ray crystallography. It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F). However, corrosion-resistance is not quantifiable in absolute terms; although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable, whereas gold dust is not flammable but can be attacked by substances that iridium resists, such as aqua regia.

Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

Osmium is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element. When experimentally measured using X-ray crystallography, it has a density of 22.59 g/cm3. Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.

Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely-used stain in electron microscopy.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Dimethylaniline monooxygenase [N-oxide-forming] 4 is an enzyme that in humans is encoded by the FMO4 gene.

Iridium hexafluoride, also iridium(VI) fluoride, (IrF6) is a compound of iridium and fluorine and one of the seventeen known binary hexafluorides. It is one of only a few compounds with iridium in the oxidation state +6.
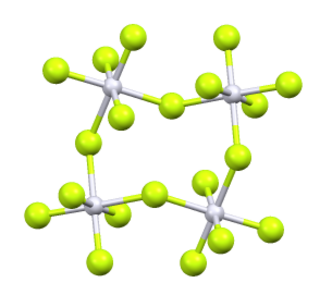
Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.
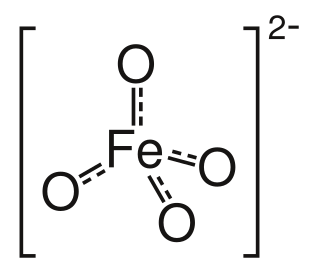
High-valent iron commonly denotes compounds and intermediates in which iron is found in a formal oxidation state > 3 that show a number of bonds > 6 with a coordination number ≤ 6. The term is rather uncommon for hepta-coordinate compounds of iron. It has to be distinguished from the terms hypervalent and hypercoordinate, as high-valent iron compounds neither necessarily violate the 18-electron rule nor necessarily show coordination numbers > 6. The ferrate(VI) ion [FeO4]2− was the first structure in this class synthesized. The synthetic compounds discussed below contain highly oxidized iron in general, as the concepts are closely related.
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds are molecular, i.e. discrete in contrast to the polymeric, dense nitride materials that are useful in materials science. The distinction between the molecular and solid-state polymers is not always very clear as illustrated by the materials Li6MoN4 and more condensed derivatives such as Na3MoN3. Transition metal nitrido complexes have attracted interest in part because it is assumed that nitrogen fixation proceeds via nitrido intermediates. Nitrido complexes have long been known, the first example being salts of [OsO3N]−, described in the 19th century.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand. The -PH2 ion or ligand is also called phosphanide or phosphido ligand.
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +3, and +4. Well-characterized compounds containing iridium in the +6 oxidation state include IrF6 and the oxides Sr2MgIrO6 and Sr2CaIrO6. iridium(VIII) oxide was generated under matrix isolation conditions at 6 K in argon. The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO4]+.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.

Osmium octafluoride is an inorganic chemical compound of osmium metal and fluorine with the chemical formula OsF8. Some sources consider it to be a still hypothetical compound. An early report of the synthesis of OsF8 was much later shown to be a mistaken identification of OsF6. Theoretical analysis indicates OsF8 would have an approximately square antiprismatic molecular geometry.
References
- 1 2 3 4 5 Riedel, S.; Kaupp, M. (30 Jul 2009). "The highest oxidation states of the transition metal elements". Coordination Chemistry Reviews. 253 (5–6): 606–624. doi:10.1016/j.ccr.2008.07.014.
- ↑ Jianyan Lin, Ziyuan Zhao, Chunyu Liu, Jing Zhang, Xin Du, Guochun Yang, and Yanming Ma (March 13, 2019). "IrF8 Molecular Crystal under High Pressure". J. Am. Chem. Soc. 141 (13): 5409–5414. doi:10.1021/jacs.9b00069. PMID 30864432. S2CID 76664353.
{{cite journal}}: CS1 maint: multiple names: authors list (link)