
In medicine, a prosthesis, or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through physical trauma, disease, or a condition present at birth. Prostheses are intended to restore the normal functions of the missing body part. Amputee rehabilitation is primarily coordinated by a physiatrist as part of an inter-disciplinary team consisting of physiatrists, prosthetists, nurses, physical therapists, and occupational therapists. Prostheses can be created by hand or with computer-aided design (CAD), a software interface that helps creators design and analyze the creation with computer-generated 2-D and 3-D graphics as well as analysis and optimization tools.
An evisceration is the removal of the eye's contents, leaving the scleral shell and extraocular muscles intact. The procedure is usually performed to reduce pain or improve cosmesis in a blind eye, as in cases of endophthalmitis unresponsive to antibiotics. An ocular prosthetic can be fitted over the eviscerated eye in order to improve cosmesis.

In anatomy, the orbit is the cavity or socket/hole of the skull in which the eye and its appendages are situated. "Orbit" can refer to the bony socket, or it can also be used to imply the contents. In the adult human, the volume of the orbit is 30 millilitres, of which the eye occupies 6.5 ml. The orbital contents comprise the eye, the orbital and retrobulbar fascia, extraocular muscles, cranial nerves II, III, IV, V, and VI, blood vessels, fat, the lacrimal gland with its sac and duct, the eyelids, medial and lateral palpebral ligaments, cheek ligaments, the suspensory ligament, septum, ciliary ganglion and short ciliary nerves.

Eye surgery, also known as ophthalmic surgery or ocular surgery, is surgery performed on the eye or its adnexa. Eye surgery is part of ophthalmology and is performed by an ophthalmologist or eye surgeon. The eye is a fragile organ, and requires due care before, during, and after a surgical procedure to minimize or prevent further damage. An eye surgeon is responsible for selecting the appropriate surgical procedure for the patient, and for taking the necessary safety precautions. Mentions of eye surgery can be found in several ancient texts dating back as early as 1800 BC, with cataract treatment starting in the fifth century BC. It continues to be a widely practiced class of surgery, with various techniques having been developed for treating eye problems.

Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant, that is, a hip prosthesis. Hip replacement surgery can be performed as a total replacement or a hemi/semi(half) replacement. Such joint replacement orthopaedic surgery is generally conducted to relieve arthritis pain or in some hip fractures. A total hip replacement consists of replacing both the acetabulum and the femoral head while hemiarthroplasty generally only replaces the femoral head. Hip replacement is one of the most common orthopaedic operations, though patient satisfaction varies widely. Approximately 58% of total hip replacements are estimated to last 25 years. The average cost of a total hip replacement in 2012 was $40,364 in the United States, and about $7,700 to $12,000 in most European countries.

Duane syndrome is a congenital rare type of strabismus most commonly characterized by the inability of the eye to move outward. The syndrome was first described by ophthalmologists Jakob Stilling (1887) and Siegmund Türk (1896), and subsequently named after Alexander Duane, who discussed the disorder in more detail in 1905.

The lateral rectus muscle is a muscle on the lateral side of the eye in the orbit. It is one of six extraocular muscles that control the movements of the eye. The lateral rectus muscle is responsible for lateral movement of the eyeball, specifically abduction. Abduction describes the movement of the eye away from the midline, allowing the eyeball to move horizontally in the lateral direction, bringing the pupil away from the midline of the body.
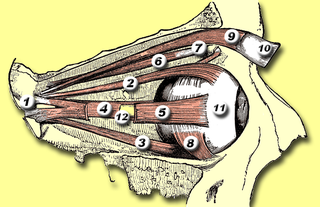
The medial rectus muscle is a muscle in the orbit near the eye. It is one of the extraocular muscles. It originates from the common tendinous ring, and inserts into the anteromedial surface of the eye. It is supplied by the inferior division of the oculomotor nerve (III). It rotates the eye medially (adduction).

The extraocular muscles, or extrinsic ocular muscles, are the seven extrinsic muscles of the eye in humans and other animals. Six of the extraocular muscles, the four recti muscles, and the superior and inferior oblique muscles, control movement of the eye. The other muscle, the levator palpebrae superioris, controls eyelid elevation. The actions of the six muscles responsible for eye movement depend on the position of the eye at the time of muscle contraction.

Enucleation is the removal of the eye that leaves the eye muscles and remaining orbital contents intact. This type of ocular surgery is indicated for a number of ocular tumors, in eyes that have sustained severe trauma, and in eyes that are otherwise blind and painful.

Joint replacement is a procedure of orthopedic surgery known also as arthroplasty, in which an arthritic or dysfunctional joint surface is replaced with an orthopedic prosthesis. Joint replacement is considered as a treatment when severe joint pain or dysfunction is not alleviated by less-invasive therapies. Joint replacement surgery is often indicated from various joint diseases, including osteoarthritis and rheumatoid arthritis.
An ocularist specializes in the fabrication and fitting of ocular prostheses for people who have lost an eye or eyes due to trauma or illness. The fabrication process for a custom made eye typically includes taking an impression of the eye socket, shaping a plastic shell, painting the iris, and then fitting the ocular prostheses. Prefabricated ocular prostheses with different colored iris are also available. An ocularist may select the stock eye that is most closely matched to patient's iris color. However, due to better adaptation, comfort, and aesthetics, custom-made ocular prostheses are more accepted. In addition to creating the prosthetic eye, an ocularist shows the patient how to care for and handle the prosthesis.
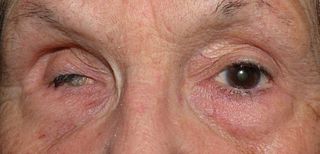
Phthisis bulbi is a shrunken, non-functional eye. It may result from severe eye disease, inflammation or injury, or it may represent a complication of eye surgery. Treatment options include insertion of a prosthesis, which may be preceded by enucleation of the eye.

Tenon's capsule, also known as the Tenon capsule, fascial sheath of the eyeball or the fascia bulbi, is a thin membrane which envelops the eyeball from the optic nerve to the corneal limbus, separating it from the orbital fat and forming a socket in which it moves.
Oculoplastics, or oculoplastic surgery, includes a wide variety of surgical procedures that deal with the orbit, eyelids, tear ducts, and the face. It also deals with the reconstruction of the eye and associated structures.

An eye neoplasm is a tumor of the eye. A rare type of tumor, eye neoplasms can affect all parts of the eye, and can either be benign or malignant (cancerous), in which case it is known as eye cancer. Eye cancers can be primary or metastatic cancer. The two most common cancers that spread to the eye from another organ are breast cancer and lung cancer. Other less common sites of origin include the prostate, kidney, thyroid, skin, colon and blood or bone marrow.

Anophthalmia is the medical term for the absence of one or both eyes. Both the globe and the ocular tissue are missing from the orbit. The absence of the eye will cause a small bony orbit, a constricted mucosal socket, short eyelids, reduced palpebral fissure and malar prominence. Genetic mutations, chromosomal abnormalities, and prenatal environment can all cause anophthalmia. Anophthalmia is an extremely rare disease and is mostly rooted in genetic abnormalities. It can also be associated with other syndromes.

An orbital blowout fracture is a traumatic deformity of the orbital floor or medial wall that typically results from the impact of a blunt object larger than the orbital aperture, or eye socket. Most commonly this results in a herniation of orbital contents through the orbital fractures. The proximity of maxillary and ethmoidal sinus increases the susceptibility of the floor and medial wall for the orbital blowout fracture in these anatomical sites. Most commonly, the inferior orbital wall, or the floor, is likely to collapse, because the bones of the roof and lateral walls are robust. Although the bone forming the medial wall is the thinnest, it is buttressed by the bone separating the ethmoidal air cells. The comparatively thin bone of the floor of the orbit and roof of the maxillary sinus has no support and so the inferior wall collapses mostly. Therefore, medial wall blowout fractures are the second-most common, and superior wall, or roof and lateral wall, blowout fractures are uncommon and rare, respectively. They are characterized by double vision, sunken ocular globes, and loss of sensation of the cheek and upper gums from infraorbital nerve injury.

Paul Wilhelm Richter was a South African chemist and principal researcher involved in bioceramic research activities. He is most widely known for his development of the bioceramic hydroxyapatite orbital implant.
Minimally invasive strabismus surgery (MISS) is a technique in strabismus surgery that uses smaller incisions than the classical surgical approach to correct the condition, thus minimizing tissue disruption. The technique was introduced by Swiss ophthalmologist Daniel Mojon around 2007, after the Belgian ophthalmologist Marc Gobin described the idea in 1994 in a French-language textbook.




















