
Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Sulfuric acid or sulphuric acid, known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

Patina is a thin layer that variously forms on the surface of copper, brass, bronze, and similar metals and metal alloys, or certain stones and wooden furniture, or any similar acquired change of a surface through age and exposure.
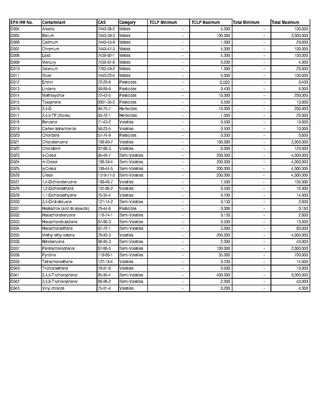
Toxicity characteristic leaching procedure (TCLP) is a soil sample extraction method for chemical analysis employed as an analytical method to simulate leaching through a landfill. The testing methodology is used to determine if a waste is characteristically hazardous, i.e., classified as one of the "D" listed wastes by the U.S. Environmental Protection Agency (EPA). The extract is analyzed for substances appropriate to the protocol.
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

The sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.

Piranha solution, also known as piranha etch, is a mixture of sulfuric acid and hydrogen peroxide. The resulting mixture is used to clean organic residues off substrates, for example silicon wafers. Because the mixture is a strong oxidizing agent, it will decompose most organic matter, and it will also hydroxylate most surfaces, making them highly hydrophilic (water-compatible). This means the solution can also easily dissolve fabric and skin, potentially causing severe damage and chemical burns in case of inadvertent contact. It is named after the piranha fish due to its tendency to rapidly dissolve and ’consume’ organic materials through vigorous chemical reactions.

Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds.

Conservation and restoration of metals is the activity devoted to the protection and preservation of historical and archaeological objects made partly or entirely of metal. In it are included all activities aimed at preventing or slowing deterioration of items, as well as improving accessibility and readability of the objects of cultural heritage. Despite the fact that metals are generally considered as relatively permanent and stable materials, in contact with the environment they deteriorate gradually, some faster and some much slower. This applies especially to archaeological finds.
Conservation and restoration of movable cultural property is a term used to denote the conservation of movable cultural property items in libraries, archives, museums and private collections. Conservation encompasses all the actions taken toward the long-term preservation of cultural heritage. Activities include examination, documentation, treatment, and preventive care, which is supported by research and education. Object conservation is specifically the actions taken to preserve and restore cultural objects. The objects span a wide range of materials from a variety of cultures, time periods, and functions. Object conservation can be applied to both art objects and artifacts. Conservation practice aims to prevent damage from occurring, a process known as 'preventive conservation'. The purpose of preventive conservation is to maintain, and where possible enhance, the condition of an object, as well as managing deterioration risks, such as handling and environmental conditions. Historically, object conservation was focused on the category of fine arts but now many different types of objects are conserved. Each type of object material, typically denoted by organic or inorganic then the specific medium, requires a specialized professional conservator and often requires collaborative work between museum staff, scientists, and conservators.

The conservation and restoration of silver objects is an activity dedicated to the preservation and protection of objects of historical and personal value made from silver. When applied to cultural heritage this activity is generally undertaken by a conservator-restorer.

Collection maintenance is an area of collections management that consists of the day-to-day hands on care of collections and cultural heritage. The primary goal of collections maintenance or preventive conservation is to prevent further decay of cultural heritage by ensuring proper storage and upkeep including performing regular housekeeping of the spaces and objects and monitoring and controlling storage and gallery environments. Collections maintenance is part of the risk management field of collections management. The professionals most involved with collections maintenance include collection managers, registrars, and archivists, depending on the size and scope of the institution. Collections maintenance takes place in two primary areas of the museum: storage areas and display areas.

The conservation and restoration of books, manuscripts, documents, and ephemera is an activity dedicated to extending the life of items of historical and personal value made primarily from paper, parchment, and leather. When applied to cultural heritage, conservation activities are generally undertaken by a conservator. The primary goal of conservation is to extend the lifespan of the object as well as maintaining its integrity by keeping all additions reversible. Conservation of books and paper involves techniques of bookbinding, restoration, paper chemistry, and other material technologies including preservation and archival techniques.

An Objects conservator is a professional, working in a museum setting or private practice, that specializes in the conservation of three-dimensional works. They undergo specialized education, training, and experience that allows them to formulate and implement preventive strategies and invasive treatment protocols to preserve cultural property for the future. Objects conservators typically specialize in one type of material or class of cultural property, including metals, archaeological artifacts, ethnographic artifacts, glass, and ceramic art. Objects conservation presents many challenges due to their three-dimensional form and composite nature.

A conservation scientist is a museum professional who works in the field of conservation science and whose focus is on the research of cultural heritage through scientific inquiry. Conservation scientists conduct applied scientific research and techniques to determine the material, chemical, and technical aspects of cultural heritage. The technical information conservation scientists gather is then used by conservator and curators to decide the most suitable conservation treatments for the examined object and/or adds to our knowledge about the object by providing answers about the material composition, fabrication, authenticity, and previous restoration treatments.
William Andrew Oddy, is a former Keeper of Conservation at the British Museum, notable for his publications on artefact conservation and numismatics, and for the development of the Oddy test. In 1996 he was awarded the Forbes Prize "for outstanding work in the field of conservation" by the International Institute for Conservation, and gave the attendant Forbes Lecture that year in Copenhagen. He retired in 2002 and was appointed as an Officer of the Order of the British Empire the same year.

Flowers of sulfur (FOS) testing was developed to determine the porosity of metallic coatings susceptible to sulfur induced corrosion [see below ASTM B809-95(2018)]. Applicable substrates are silver, copper, copper alloys and any other metal or metal alloy with which sulfur will react. For porosity testing, coatings can be single or multiple layers of any metal that is not corroded and sealed by a self-limiting reaction in the reducing sulfur environment of the FOS test. The simplest recommended technique is to identify any porosity of the coating as revealed by the presence of surface spots. These surface spots form where the environmental sulfur has penetrated and reacted with the base metal, producing a metal sulfide. Chalcocite, copper (I) sulfide is dark-grey to black. Silver (I) sulfide is also grey-black.

The 'ten agents of deterioration' are a conceptual framework developed by the Canadian Conservation Institute (CCI) used to categorise the major causes of change, loss or damage to cultural heritage objects. Also referred to as the 'agents of change', the framework was first developed in the late 1980s and early 1990s. The defined agents reflect and systematise the main chemical and physical deterioration pathways to which most physical material is subject. They are a major influence on the applied practice of conservation, restoration, and collection management, finding particular use in risk management for cultural heritage collections.


















