
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Most commonly, protein phosphorylation is catalyzed by protein kinases, ultimately resulting in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Visual phototransduction is the sensory transduction process of the visual system by which light is detected to yield nerve impulses in the rod cells and cone cells in the retina of the eye in humans and other vertebrates. It relies on the visual cycle, a sequence of biochemical reactions in which a molecule of retinal bound to opsin undergoes photoisomerization, initiates a cascade that signals detection of the photon, and is indirectly restored to its photosensitive isomer for reuse. Phototransduction in some invertebrates such as fruit flies relies on similar processes.
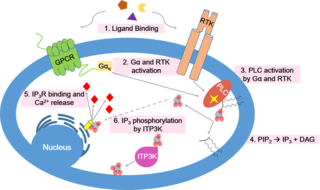
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.
The sodium-calcium exchanger (often denoted Na+/Ca2+ exchanger, exchange protein, or NCX) is an antiporter membrane protein that removes calcium from cells. It uses the energy that is stored in the electrochemical gradient of sodium (Na+) by allowing Na+ to flow down its gradient across the plasma membrane in exchange for the countertransport of calcium ions (Ca2+). A single calcium ion is exported for the import of three sodium ions. The exchanger exists in many different cell types and animal species. The NCX is considered one of the most important cellular mechanisms for removing Ca2+.
p38 mitogen-activated protein kinases are a class of mitogen-activated protein kinases (MAPKs) that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy. Persistent activation of the p38 MAPK pathway in muscle satellite cells due to ageing, impairs muscle regeneration.
Osmolytes are low-molecular-weight organic compounds that influence the properties of biological fluids. Osmolytes are a class of organic molecules that play a significant role in regulating osmotic pressure and maintaining cellular homeostasis in various organisms, particularly in response to environmental stressors. Their primary role is to maintain the integrity of cells by affecting the viscosity, melting point, and ionic strength of the aqueous solution. When a cell swells due to external osmotic pressure, membrane channels open and allow efflux of osmolytes carrying water, restoring normal cell volume.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Nuclear factor of activated T-cells 5, also known as NFAT5 and sometimes TonEBP, is a human gene that encodes a transcription factor that regulates the expression of genes involved in the osmotic stress.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.
Osmoprotectants or compatible solutes are small organic molecules with neutral charge and low toxicity at high concentrations that act as osmolytes and help organisms survive extreme osmotic stress. Osmoprotectants can be placed in three chemical classes: betaines and associated molecules, sugars and polyols, and amino acids. These molecules accumulate in cells and balance the osmotic difference between the cell's surroundings and the cytosol. In plants, their accumulation can increase survival during stresses such as drought. In extreme cases, such as in bdelloid rotifers, tardigrades, brine shrimp, and nematodes, these molecules can allow cells to survive being completely dried out and let them enter a state of suspended animation called cryptobiosis.

Acid-sensing ion channels (ASICs) are neuronal voltage-insensitive sodium channels activated by extracellular protons permeable to Na+. ASIC1 also shows low Ca2+ permeability. ASIC proteins are a subfamily of the ENaC/Deg superfamily of ion channels. These genes have splice variants that encode for several isoforms that are marked by a suffix. In mammals, acid-sensing ion channels (ASIC) are encoded by five genes that produce ASIC protein subunits: ASIC1, ASIC2, ASIC3, ASIC4, and ASIC5. Three of these protein subunits assemble to form the ASIC, which can combine into both homotrimeric and heterotrimeric channels typically found in both the central nervous system and peripheral nervous system. However, the most common ASICs are ASIC1a and ASIC1a/2a and ASIC3. ASIC2b is non-functional on its own but modulates channel activity when participating in heteromultimers and ASIC4 has no known function. On a broad scale, ASICs are potential drug targets due to their involvement in pathological states such as retinal damage, seizures, and ischemic brain injury.
Plants are exposed to many stress factors such as disease, temperature changes, herbivory, injury and more. Therefore, in order to respond or be ready for any kind of physiological state, they need to develop some sort of system for their survival in the moment and/or for the future. Plant communication encompasses communication using volatile organic compounds, electrical signaling, and common mycorrhizal networks between plants and a host of other organisms such as soil microbes, other plants, animals, insects, and fungi. Plants communicate through a host of volatile organic compounds (VOCs) that can be separated into four broad categories, each the product of distinct chemical pathways: fatty acid derivatives, phenylpropanoids/benzenoids, amino acid derivatives, and terpenoids. Due to the physical/chemical constraints most VOCs are of low molecular mass, are hydrophobic, and have high vapor pressures. The responses of organisms to plant emitted VOCs varies from attracting the predator of a specific herbivore to reduce mechanical damage inflicted on the plant to the induction of chemical defenses of a neighboring plant before it is being attacked. In addition, the host of VOCs emitted varies from plant to plant, where for example, the Venus Fly Trap can emit VOCs to specifically target and attract starved prey. While these VOCs typically lead to increased resistance to herbivory in neighboring plants, there is no clear benefit to the emitting plant in helping nearby plants. As such, whether neighboring plants have evolved the capability to "eavesdrop" or whether there is an unknown tradeoff occurring is subject to much scientific debate. As related to the aspect of meaning-making, the field is also identified as phytosemiotics.
As a model organism, the Arabidopsis thaliana response to salinity is studied to aid understanding of other more economically important crops.

Sodium- and chloride-dependent betaine transporter, also known as Na(+)/Cl(-) betaine/GABA transporter (BGT-1), is a protein that in humans is encoded by the SLC6A12 gene. BGT-1 is predominantly expressed in the liver (hepatocytes). It is also expressed in the kidney where it is regulated by NFAT5 during a response to osmotic stress. Further, BGT1 is also present in the leptomeninges surrounding the brain. Deletion of the BGT1 gene in mice did not appear to have any impact on the tendency to develop epilepsy. This is to be expected considering that BGT1 is expressed at far lower levels than GAT1 and also has lower affinity for GABA. This implies that it is not likely to contribute significantly to the inactivation of the inhibitory neurotransmitter GABA.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.










