History
| | This section needs expansion. You can help by adding to it. (May 2024) |
Wilhelm Ostwald developed the process, and he patented it in 1902. [11] [12]
The Ostwald process is a chemical process used for making nitric acid (HNO3). [1] The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. [2] Historically and practically, the Ostwald process is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3).
Ammonia is converted to nitric acid in 2 stages.
The Ostwald process begins with burning ammonia. Ammonia burns in oxygen at temperature about 900 °C (1,650 °F) and pressure up to 8 standard atmospheres (810 kPa) [3] in the presence of a catalyst such as platinum gauze, alloyed with 10% rhodium to increase its strength and NO yield, platinum metal on fused silica wool, copper or nickel to form nitric oxide (nitrogen(II) oxide) and water (as steam). This catalyst is frequently replaced due to decomposition as a result of the extreme conditions which it operates under. [4] This reaction is strongly exothermic, making it a useful heat source once initiated: [5]
A number of side reactions compete with the formation of nitric oxide. Some reactions convert the ammonia to N2, such as:
This is a secondary reaction that is minimised by reducing the time the gas mixtures are in contact with the catalyst. [6] Another side reaction produces nitrous oxide:
The nitric oxide (NO) formed in the prior catalysed reaction is then cooled down from around 900˚C to roughly 250˚C to be further oxidised to nitrogen dioxide (NO2) [7] by the reaction:
2NO + O2 → 2NO2 (ΔH = -114.2 kJ/mol) [8]
The reaction:
2NO2 → N2O4 (ΔH = -57.2 kJ/mol) [9]
also occurs once the nitrogen dioxide has formed. [10]
Stage two encompasses the absorption of nitrous oxides in water and is carried out in an absorption apparatus, a plate column containing water[ citation needed ]. This gas is then readily absorbed by the water, yielding the desired product (nitric acid in a dilute form), while reducing a portion of it back to nitric oxide [5] :
The NO is recycled, and the acid is concentrated to the required strength by distillation.
This is only one of over 40 absorption reactions of nitrous oxides recorded [10] , with other common reactions including:
And, if the last step is carried out in air:
The overall reaction is the sum of the first equation, 3 times the second equation, and 2 times the last equation; all divided by 2:
Alternatively, if the last step is carried out in the air, the overall reaction is the sum of equation 1, 2 times equation 2, and equation 4; all divided by 2.
Without considering the state of the water,
| | This section needs expansion. You can help by adding to it. (May 2024) |
Wilhelm Ostwald developed the process, and he patented it in 1902. [11] [12]

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.

Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Red fuming nitric acid (RFNA) is a storable oxidizer used as a rocket propellant. It consists of 84% nitric acid, 13% dinitrogen tetroxide and 1–2% water. The color of red fuming nitric acid is due to the dinitrogen tetroxide, which breaks down partially to form nitrogen dioxide. The nitrogen dioxide dissolves until the liquid is saturated, and produces toxic fumes with a suffocating odor. RFNA increases the flammability of combustible materials and is highly exothermic when reacting with water.
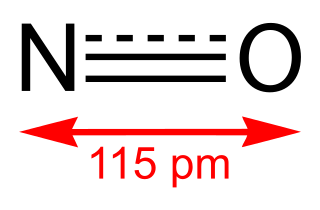
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
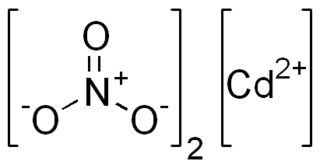
Cadmium nitrate describes any of the related members of a family of inorganic compounds with the general formula Cd(NO3)2·xH2O. The most commonly encountered form being the tetrahydrate.The anhydrous form is volatile, but the others are colourless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic. According to X-ray crystallography, the tetrahydrate features octahedral Cd2+ centers bound to six oxygen ligands.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

The Birkeland–Eyde process was one of the competing industrial processes in the beginning of nitrogen-based fertilizer production. It is a multi-step nitrogen fixation reaction that uses electrical arcs to react atmospheric nitrogen (N2) with oxygen (O2), ultimately producing nitric acid (HNO3) with water. The resultant nitric acid was then used as a source of nitrate (NO3−) in the reaction which may take place in the presence of water or another proton acceptor.

Sable Chemical Industries Limited is the sole manufacturer of ammonium nitrate (NH4NO3) in Zimbabwe.