Related Research Articles
Actinium is a chemical element; it has symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

High-temperature superconductors are defined as materials with critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at even colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first break through of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.

A pnictogen is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc).

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Bismuth strontium calcium copper oxide (BSCCO, pronounced bisko), is a type of cuprate superconductor having the generalized chemical formula Bi2Sr2Can−1CunO2n+4+x, with n = 2 being the most commonly studied compound (though n = 1 and n = 3 have also received significant attention). Discovered as a general class in 1988, BSCCO was the first high-temperature superconductor which did not contain a rare-earth element.

Calcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
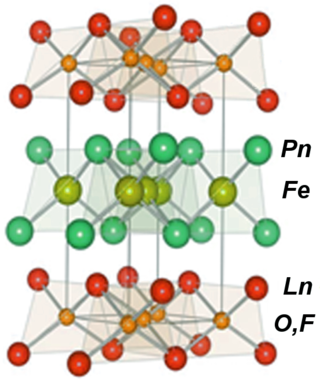
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..
In chemistry, oxypnictides are a class of materials composed of oxygen, a pnictogen and one or more other elements. Although this group of compounds has been recognized since 1995, interest in these compounds increased dramatically after the publication of the superconducting properties of LaOFeP and LaOFeAs which were discovered in 2006 and 2008. In these experiments the oxide was partly replaced by fluoride.

Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.

Bismuth oxychloride is an inorganic compound of bismuth with the formula BiOCl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. It is also known as pearl white.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Oxyphosphides are chemical compounds formally containing the group PO, with one phosphorus and one oxygen atom. The phosphorus and oxygen are not bound together as in phosphates or phosphine oxides, instead they are bound separately to the cations (metals), and could be considered as a mixed phosphide-oxide compound. So a compound with OmPn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxy-pnictide compounds.
Oxyarsenides or arsenide oxides are chemical compounds formally containing the group AsO, with one arsenic and one oxygen atom. The arsenic and oxygen are not bound together as in arsenates or arsenites, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed arsenide-oxide compound. So a compound with OmAsn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.

An arsenate arsenite is a chemical compound or salt that contains arsenate and arsenite anions (AsO33- and AsO43-). These are mixed anion compounds or mixed valence compounds. Some have third anions. Most known substances are minerals, but a few artificial arsenate arsenite compounds have been made. Many of the minerals are in the Hematolite Group.

Neodymium bismuthide or Bismuth-Neodymium is a binary inorganic compound of neodymium and bismuth with the formula NdBi. It forms crystals.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).

Bismuth compounds are compounds containing the element bismuth (Bi). Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements.

Praseodymium bismuthide is a binary inorganic compound of praseodymium and bismuth with the chemical formula of PrBi. It forms crystals.
References
- 1 2 3 4 5 Forbes, Scott; Mozharivskyj, Yurij (2017-11-28). "Rare-Earth Pnictide Oxides ( RE ,Ca) m Pn n O m ( Pn = Sb, Bi): A Review of Crystal Structures, Chemistry, Compositions, and Physical Properties". Chemistry of Materials. 29 (22): 9605–9612. doi:10.1021/acs.chemmater.7b03996. ISSN 0897-4756.
- ↑ Amano, Shinsaku; Yamane, Hisanori (August 2016). "Synthesis and crystal structure analysis of titanium bismuthide oxide, Ti8BiO7". Journal of Alloys and Compounds. 675: 377–380. doi:10.1016/j.jallcom.2016.03.096.
- 1 2 Zhai, Hui-Fei; Jing, Yi-Shuai; Zhang, Pan; Lin, Bo; Song, Jia-Ming; Hu, Peng; Li, Bai-Zhuo; Du, Jian-Hua; Jiao, Zhi-Wei; Cao, Guang-Han (2022-11-17). "Structure and Physical Properties of the Layered Titanium-Based Pnictide Oxides (EuF) 2 Ti 2 Pn 2 O (Pn = Sb, Bi)". Inorganic Chemistry. 61 (48): 19232–19239. doi:10.1021/acs.inorgchem.2c02895. ISSN 0020-1669. PMID 36395178. S2CID 253627945.
- ↑ Hosono, Hideo; Tanabe, Keiichi; Takayama-Muromachi, Eiji; Kageyama, Hiroshi; Yamanaka, Shoji; Kumakura, Hiroaki; Nohara, Minoru; Hiramatsu, Hidenori; Fujitsu, Satoru (2015-05-08). "Exploration of new superconductors and functional materials, and fabrication of superconducting tapes and wires of iron pnictides". Science and Technology of Advanced Materials. 16 (3): 033503. arXiv: 1505.02240 . Bibcode:2015STAdM..16c3503H. doi:10.1088/1468-6996/16/3/033503. ISSN 1468-6996. PMC 5099821 . PMID 27877784.
- ↑ Kozhevnikov, V. L.; Leonidova, O. N.; Ivanovskii, A. L.; Shein, I. R.; Goshchitskii, B. N.; Kar’kin, A. E. (5 February 2009). "New superconductor with a layered crystal structure: Nickel oxybismuthide LaO1−δNiBi". JETP Letters. 87 (11): 649–651. doi:10.1134/S002136400811012X. S2CID 120451483.
- ↑ Benz, R. (1971-04-01). "Ce 2 O 2 Sb and Ce 2 O 2 Bi crystal structure". Acta Crystallographica Section B. 27 (4): 853–854. Bibcode:1971AcCrB..27..853B. doi:10.1107/S0567740871003066. ISSN 0567-7408.
- 1 2 3 4 Nuss, Jürgen; Jansen, Martin (March 2012). "On the Rare Earth Metal Bismuthide Oxides RE2BiO2 (RE = Nd, Tb, Dy, Ho)". Zeitschrift für anorganische und allgemeine Chemie. 638 (3–4): 611–613. doi:10.1002/zaac.201100529.
- 1 2 Kim, Heejung; Kang, Chang-Jong; Kim, Kyoo; Shim, J. H.; Min, B. I. (2016-03-09). "Suppression of the charge density wave instability in R 2 O 2 Bi ( R = La, Er) due to large spin-orbit coupling" (PDF). Physical Review B. 93 (12): 125116. Bibcode:2016PhRvB..93l5116K. doi:10.1103/PhysRevB.93.125116. ISSN 2469-9950.
- 1 2 3 4 5 6 Mizoguchi, Hiroshi; Hosono, Hideo (2011-03-02). "A Metal−Insulator Transition in R 2 O 2 Bi with an Unusual Bi 2− Square Net (R = Rare Earth or Y)". Journal of the American Chemical Society. 133 (8): 2394–2397. doi:10.1021/ja111015p. ISSN 0002-7863. PMID 21302922.
- ↑ Nuss, Jürgen; Wedig, Ulrich; Jansen, Martin (November 2011). "Geometric Variations and Electron Localizations in Intermetallics: The Case of La2Sb Type Compounds". Zeitschrift für anorganische und allgemeine Chemie. 637 (13): 1975–1981. doi:10.1002/zaac.201100331.
- ↑ Xia, Sheng-Qing; Bobev, Svilen (2010-12-15). "Dibarium tricadmium bismuthide(-I,-III) oxide, Ba 2 Cd 3−δ Bi 3 O". Acta Crystallographica Section E. 66 (12): i81. Bibcode:2010AcCrE..66I..81X. doi:10.1107/S1600536810046283. ISSN 1600-5368. PMC 3011431 . PMID 21589204.
- 1 2 Forbes, Scott; Yuan, Fang; Kosuda, Kosuke; Kolodiazhnyi, Taras; Mozharivskyj, Yurij (January 2016). "Synthesis, crystal structure, and physical properties of the Gd3BiO3 and Gd8Bi3O8 phases". Journal of Solid State Chemistry. 233: 252–258. Bibcode:2016JSSCh.233..252F. doi:10.1016/j.jssc.2015.10.004.
- ↑ Seaborg, G. T.; Katz, Joseph J.; Morss, L. R. (2012). The Chemistry of the Actinide Elements. Springer Science & Business Media. p. 972. ISBN 978-94-009-3155-8.
- ↑ Seaborg, G. T.; Katz, Joseph J.; Morss, L. R. (2012). The Chemistry of the Actinide Elements. Springer Science & Business Media. p. 902. ISBN 978-94-009-3155-8.