
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen each atom has one proton, one electron, and no neutrons.

Photosynthesis is a biological process used by many cellular organisms to convert light energy into chemical energy, which is stored in organic compounds that can later be metabolized through cellular respiration to fuel the organism's activities. The term usually refers to oxygenic photosynthesis, where oxygen is produced as a byproduct, and some of the chemical energy produced is stored in carbohydrate molecules such as sugars, starch and cellulose, which are synthesized from endergonic reaction of carbon dioxide with water. Most plants, algae and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the biological energy necessary for complex life on Earth.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

Redox is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
In chemistry, a reducing agent is a chemical species that "donates" an electron to an electron recipient. Examples of substances that are common reducing agents include the alkali metals, formic acid, oxalic acid, and sulfite compounds.
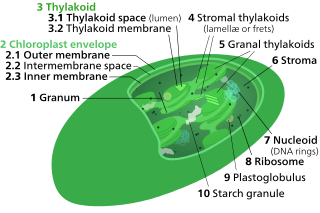
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.

Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII.

Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.

In the process of photosynthesis, the phosphorylation of ADP to form ATP using the energy of sunlight is called photophosphorylation. Cyclic photophosphorylation occurs in both aerobic and anaerobic conditions, driven by the main primary source of energy available to living organisms, which is sunlight. All organisms produce a phosphate compound, ATP, which is the universal energy currency of life. In photophosphorylation, light energy is used to pump protons across a biological membrane, mediated by flow of electrons through an electron transport chain. This stores energy in a proton gradient. As the protons flow back through an enzyme called ATP synthase, ATP is generated from ADP and inorganic phosphate. ATP is essential in the Calvin cycle to assist in the synthesis of carbohydrates from carbon dioxide and NADPH.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach circa 2,800°C.
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule.

A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical energy.

Biohydrogen is H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic microorganisms are capable to produce H2 directly from water splitting using light as energy source.
Dioxygen plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis of water during photosynthesis in cyanobacteria, green algae, and plants. During oxidative phosphorylation in cellular respiration, oxygen is reduced to water, thus closing the biological water-oxygen redox cycle.

Light-dependent reactions is jargon for certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and the second occurs at photosystem I (PSI),
Water oxidation is one of the half reactions of water splitting:

Water oxidation catalysis (WOC) is the acceleration (catalysis) of the conversion of water into oxygen and protons:











