
Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.

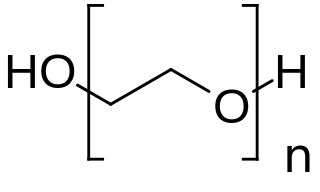
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts, or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerns such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. A comprehensive classification system based on structure-property-application relationships has been proposed for excipients used in parenteral medications.

Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its manufacture employs the same method today. The EPA estimates 5.7% of LDPE is recycled in the United States. Despite competition from more modern polymers, LDPE continues to be an important plastic grade. In 2013 the worldwide LDPE market reached a volume of about US$33 billion.
In biotechnology, polymersomes are a class of artificial vesicles, tiny hollow spheres that enclose a solution. Polymersomes are made using amphiphilic synthetic block copolymers to form the vesicle membrane, and have radii ranging from 50 nm to 5 µm or more. Most reported polymersomes contain an aqueous solution in their core and are useful for encapsulating and protecting sensitive molecules, such as drugs, enzymes, other proteins and peptides, and DNA and RNA fragments. The polymersome membrane provides a physical barrier that isolates the encapsulated material from external materials, such as those found in biological systems.
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is also used to empty the bowels before a colonoscopy. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks. It is also used as an excipient.
Polypure is a Norwegian company that manufactures and markets monodisperse PEG derivatives for applications in nanotechnology, biotechnology and in pharmaceutical sciences.
Tocofersolan (INN) or tocophersolan, also referred to as TPGS, is a synthetic water-soluble version of vitamin E. Natural forms of vitamin E are fat soluble, but not water-soluble. Tocofersolan is polyethylene glycol derivative of α-tocopherol that enables water solubility.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
The behavior of quantum dots (QDs) in solution and their interaction with other surfaces is of great importance to biological and industrial applications, such as optical displays, animal tagging, anti-counterfeiting dyes and paints, chemical sensing, and fluorescent tagging. However, unmodified quantum dots tend to be hydrophobic, which precludes their use in stable, water-based colloids. Furthermore, because the ratio of surface area to volume in a quantum dot is much higher than for larger particles, the thermodynamic free energy associated with dangling bonds on the surface is sufficient to impede the quantum confinement of excitons. Once solubilized by encapsulation in either a hydrophobic interior micelle or a hydrophilic exterior micelle, the QDs can be successfully introduced into an aqueous medium, in which they form an extended hydrogel network. In this form, quantum dots can be utilized in several applications that benefit from their unique properties, such as medical imaging and thermal destruction of malignant cancers.
Ultra-low fouling is a rating of a surface's ability to shed potential contamination. Surfaces are prone to contamination, which is a phenomenon known as fouling. Unwanted adsorbates caused by fouling change the properties of a surface, which is often counter-productive to the function of that surface. Consequently, a necessity for anti-fouling surfaces has arisen in many fields: blocked pipes inhibit factory productivity, biofouling increases fuel consumption on ships, medical devices must be kept sanitary, etc. Although chemical fouling inhibitors, metallic coatings, and cleaning processes can be used to reduce fouling, non-toxic surfaces with anti-fouling properties are ideal for fouling prevention. To be considered effective, an ultra-low fouling surface must be able to repel and withstand the accumulation of detrimental aggregates down to less than 5 ng/cm2. A recent surge of research has been conducted to create these surfaces in order to benefit the biological, nautical, mechanical, and medical fields.
Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
Lysozyme PEGylation is the covalent attachment of Polyethylene glycol (PEG) to Lysozyme, which is one of the most widely investigated PEGylated proteins.








