Salvinia molesta, commonly known as giant salvinia, or as kariba weed after it infested a large portion of Lake Kariba between Zimbabwe and Zambia, is an aquatic fern, native to south-eastern Brazil. It is a free floating plant that does not attach to the soil, but instead remains buoyant on the surface of a body of water. The fronds are 0.5–4 cm long and broad, with a bristly surface caused by the hair-like strands that join at the end to form eggbeater shapes. They are used to provide a waterproof covering. These fronds are produced in pairs also with a third modified root-like frond that hangs in the water. It has devastated countless lakes throughout the United States, including Caddo Lake.

Salvinia, a genus in the family Salviniaceae, is a floating fern named in honor of Anton Maria Salvini, a 17th-century Italian scientist. Watermoss is a common name for Salvinia. The genus was published in 1754 by Jean-François Séguier, in his description of the plants found round Verona, Plantae Veronenses Twelve species are recognized, at least three of which are believed to be hybrids, in part because their sporangia are found to be empty.

Gingerol, properly as [6]-gingerol, is a phenol phytochemical compound found in fresh ginger that activates spice receptors on the tongue. Molecularly, gingerol is a relative of capsaicin and piperine, the compounds which are alkaloids, though the bioactive pathways are unconnected. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.
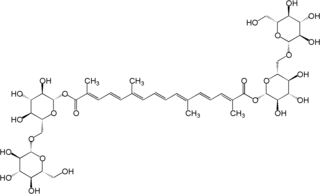
Crocin is a carotenoid chemical compound that is found in the flowers crocus and gardenia. Crocin is the chemical primarily responsible for the color of saffron.
CD120 can refer to two members of the tumor necrosis factor receptor superfamily: tumor necrosis factor receptor 1 (TNFR1) and tumor necrosis factor receptor 2 (TNFR2).
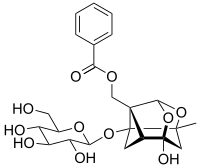
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus and in the aquatic fern Salvinia molesta. It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound. The colorless solid occurs naturally, being formed via the shikimate pathway. It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria. 2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds through amide bonds. A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsipeptide of serine.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.

Caffeic acid phenethyl ester (CAPE) is a natural phenolic chemical compound. It is the ester of caffeic acid and phenethyl alcohol.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

1,2,3,4,6-Pentagalloylglucose is the pentagallic acid ester of glucose. It is a gallotannin and the precursor of ellagitannins.
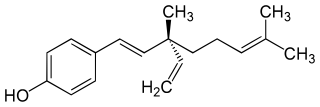
Bakuchiol is a meroterpene in the class terpenophenol.

Samea multiplicalis, the salvinia stem-borer moth, is an aquatic moth commonly found in freshwater habitats from the southern United States to Argentina, as well as in Australia where it was introduced in 1981. Salvinia stem-borer moths lay their eggs on water plants like Azolla caroliniana, Pistia stratiotes, and Salvinia rotundifolia. Larval feeding on host plants causes plant death, which makes S. multiplicalis a good candidate for biological control of weedy water plants like Salvinia molesta, an invasive water fern in Australia. However, high rates of parasitism in the moth compromise its ability to effectively control water weeds. S. multiplicalis larvae are a pale yellow to green color, and adults develop tan coloration with darker patterning. The lifespan, from egg to the end of adulthood is typically three to four weeks. The species was first described by Achille Guenée in 1854.

Salvinia minima is a species of aquatic, floating fern that grows on the surface of still waterways. It is usually referred to as common salvinia or water spangles. Salvinia minima is native to South America, Mesoamerica, and the West Indies and was introduced to the United States in the 1920s-1930s. It is classified as an invasive species internationally and can be detrimental to native ecosystems. This species is similar to but should not be confused with giant salvinia, Salvinia molesta.
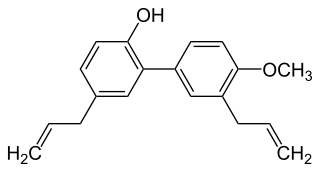
4-O-Methylhonokiol is a neolignan, a type of phenolic compound. It is found in the bark of Magnolia grandiflora and in M. virginiana flowers.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.

The head-twitch response (HTR) is a rapid side-to-side head movement that occurs in mice and rats after the serotonin 5-HT2A receptor is activated. The prefrontal cortex may be the neuroanatomical locus mediating the HTR. Many serotonergic hallucinogens, including lysergic acid diethylamide (LSD), induce the head-twitch response, and so the HTR is used as a behavioral model of hallucinogen effects. However while there is generally a good correlation between compounds that induce head twitch in mice and compounds that are hallucinogenic in humans, it is unclear whether the head twitch response is primarily caused by 5-HT2A receptors, 5-HT2C receptors or both, though recent evidence shows that the HTR is mediated by the 5-HT2A receptor and modulated by the 5-HT2C receptor. Also, the effect can be non-specific, with head twitch responses also produced by some drugs that do not act through 5-HT2 receptors, such as phencyclidine, yohimbine, atropine and cannabinoid receptor antagonists. As well, compounds such as 5-HTP, fenfluramine, 1-Methylpsilocin, Ergometrine, and 3,4-di-methoxyphenethylamine (DMPEA) can also produce head twitch and do stimulate serotonin receptors, but are not hallucinogenic in humans. This means that while the head twitch response can be a useful indicator as to whether a compound is likely to display hallucinogenic activity in humans, the induction of a head twitch response does not necessarily mean that a compound will be hallucinogenic, and caution should be exercised when interpreting such results.

Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.
The Salvinia effect describes the permanent stabilization of an air layer upon a hierarchically structured surface submerged in water. Based on biological models, biomimetic Salvinia-surfaces are used as drag reducing coatings (up to 30% reduction were previously measured on the first prototypes. When applied to a ship hull, the coating would allow the boat to float on an air-layer; reducing energy consumption and emissions. Such surfaces require an extremely water repellent super-hydrophobic surface and an elastic hairy structure in the millimeter range to entrap air while submerged. The Salvinia effect was discovered by the biologist and botanist Wilhelm Barthlott and his colleagues and has been investigated on several plants and animals since 2002. Publications and patents were published between 2006 and 2016. The best biological models are the floating ferns with highly sophisticated hierarchically structured hairy surfaces, and the back swimmers with a complex double structure of hairs and microvilli. Three of the ten known Salvinia species show a paradoxical chemical heterogeneity: hydrophilic hair tips, in addition to the super-hydrophobic plant surface, further stabilizing the air layer.