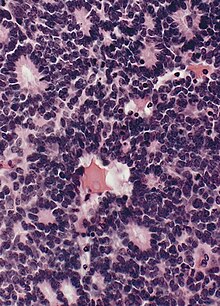
An ependymoma is a tumor that arises from the ependyma, a tissue of the central nervous system. Usually, in pediatric cases the location is intracranial, while in adults it is spinal. The common location of intracranial ependymomas is the fourth ventricle. Rarely, ependymomas can occur in the pelvic cavity.

Embryonal carcinoma is a relatively uncommon type of germ cell tumour that occurs in the ovaries and testes.
A blastoma is a type of cancer, more common in children, that is caused by malignancies in precursor cells, often called blasts. Examples are nephroblastoma, medulloblastoma, and retinoblastoma. The suffix -blastoma is used to imply a tumor of primitive, incompletely differentiated cells, e.g., chondroblastoma is composed of cells resembling the precursor of chondrocytes.

The Gleason grading system is used to help evaluate the prognosis of men with prostate cancer using samples from a prostate biopsy. Together with other parameters, it is incorporated into a strategy of prostate cancer staging which predicts prognosis and helps guide therapy. A Gleason score is given to prostate cancer based upon its microscopic appearance. Cancers with a higher Gleason score are more aggressive and have a worse prognosis. Pathological scores range from 2 to 10, with higher numbers indicating greater risks and higher mortality. The system is widely accepted and used for clinical decision making even as it is recognised that certain biomarkers, like ACP1 expression, might yield higher predictive value for future disease course.

Medulloblastoma is a common type of primary brain cancer in children. It originates in the part of the brain that is towards the back and the bottom, on the floor of the skull, in the cerebellum, or posterior fossa.

Ganglioglioma is a rare, slow-growing primary central nervous system (CNS) tumor which most frequently occurs in the temporal lobes of children and young adults

Primitive neuroectodermal tumor is a malignant (cancerous) neural crest tumor. It is a rare tumor, usually occurring in children and young adults under 25 years of age. The overall 5 year survival rate is about 53%.
Juxtaglomerular cell tumor is an extremely rare kidney tumour of the juxtaglomerular cells, with less than 100 cases reported in literature. This tumor typically secretes renin, hence the former name of reninoma. It often causes severe hypertension that is difficult to control, in adults and children, although among causes of secondary hypertension it is rare. It develops most commonly in young adults, but can be diagnosed much later in life. It is generally considered benign, but its malignant potential is uncertain.

Pineoblastoma is a malignant tumor of the pineal gland. A pineoblastoma is a supratentorial midline primitive neuroectodermal tumor. Pineoblastoma can present at any age, but is most common in young children. They account for 0.001% of all primary CNS neoplasms.

The following is a simplified (deprecated) version of the 2021 WHO classification of the tumours of the central nervous system. Currently, as of 2021, clinicians are using the WHO grade 5th edition, which incorporates recent advances in molecular pathology.
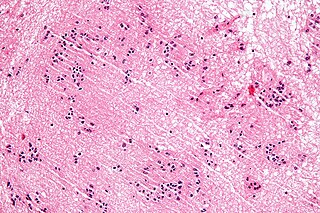
A subependymoma is a type of brain tumor; specifically, it is a rare form of ependymal tumor. They are usually in middle aged people. Earlier, they were called subependymal astrocytomas.

Medulloepithelioma is a rare, primitive, fast-growing brain tumour thought to stem from cells of the embryonic medullary cavity. Tumours originating in the ciliary body of the eye are referred to as embryonal medulloepitheliomas, or diktyomas.
Flexner is a surname. Notable people with the surname include:
Mucinous cystadenocarcinoma of the lung (MCACL) is a very rare malignant mucus-producing neoplasm arising from the uncontrolled growth of transformed epithelial cells originating in lung tissue.
Pediatric ependymomas are similar in nature to the adult form of ependymoma in that they are thought to arise from radial glial cells lining the ventricular system. However, they differ from adult ependymomas in which genes and chromosomes are most often affected, the region of the brain they are most frequently found in, and the prognosis of the patients. Children with certain hereditary diseases, such as neurofibromatosis type II (NF2), have been found to be more frequently afflicted with this class of tumors, but a firm genetic link remains to be established. Symptoms associated with the development of pediatric ependymomas are varied, much like symptoms for a number of other pediatric brain tumors including vomiting, headache, irritability, lethargy, and changes in gait. Although younger children and children with invasive tumor types generally experience less favorable outcomes, total removal of the tumors is the most conspicuous prognostic factor for both survival and relapse.

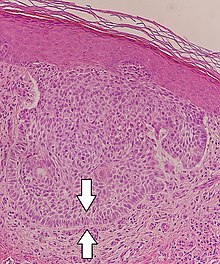
Papillary tumors of the pineal region (PTPR) were first described by A. Jouvet et al. in 2003 and were introduced in the World Health Organization (WHO) classification of Central Nervous System (CNS) in 2007. Papillary Tumors of the Pineal Region are located on the pineal gland which is located in the center of the brain. The pineal gland is located on roof of the diencephalon. It is a cone shaped structure dorsal to the midbrain tectum. The tumor appears to be derived from the specialized ependymal cells of the subcommissural organ. Papillary tumors of the central nervous system and particularly of the pineal region are very rare and so diagnosing them is extremely difficult.
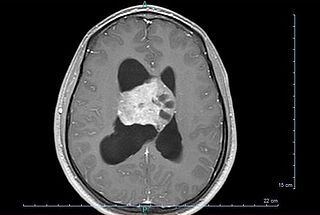
Central neurocytoma (CNC) is an extremely rare, ordinarily benign intraventricular brain tumour that typically forms from the neuronal cells of the septum pellucidum. The majority of central neurocytomas grow inwards into the ventricular system forming interventricular neurocytomas. This leads to two primary symptoms of CNCs, blurred vision and increased intracranial pressure. Treatment for a central neurocytoma typically involves surgical removal, with an approximate 1 in 5 chance of recurrence. Central neurocytomas are classified as a grade II tumor under the World Health Organization's classification of tumors of the nervous system.

A central nervous system primitive neuroectodermal tumor, often abbreviated as PNET, supratentorial PNET, or CNS-PNET, is one of the 3 types of embryonal central nervous system tumors. It is considered an embryonal tumor because it arises from cells partially differentiated or still undifferentiated from birth. Those cells are usually neuroepithelial cells, stem cells destined to turn into glia or neurons. It can occur anywhere within the spinal cord and cerebrum and can have multiple sites of origins, with a high probability of metastasis through cerebrospinal fluid (CSF).
Embryonal tumor with multilayered rosettes (ETMR) is an embryonal central nervous system tumor. It is considered an embryonal tumor because it arises from cells partially differentiated or still undifferentiated from birth, usually neuroepithelial cells, stem cells destined to turn into glia or neurons. It can occur anywhere within the brain and can have multiple sites of origins, with a high probability of metastasis through cerebrospinal fluid (CSF). Metastases outside the central nervous system have been reported, but remain rare.