
Trematoda is a class of flatworms known as flukes or trematodes. They are obligate internal parasites with a complex life cycle requiring at least two hosts. The intermediate host, in which asexual reproduction occurs, is usually a snail. The definitive host, where the flukes sexually reproduce, is a vertebrate. Infection by trematodes can cause disease in all five traditional vertebrate classes: mammals, birds, amphibians, reptiles, and fish.

Clonorchis sinensis, the Chinese liver fluke, is a liver fluke belonging to the class Trematoda, phylum Platyhelminthes. It infects fish-eating mammals, including humans. In humans, it infects the common bile duct and gall bladder, feeding on bile. It was discovered by British physician James McConnell at the Medical College Hospital in Calcutta (Kolkata) in 1874. The first description was given by Thomas Spencer Cobbold, who named it Distoma sinense. The fluke passes its lifecycle in three different hosts, namely freshwater snail as first intermediate hosts, freshwater fish as second intermediate host, and mammals as definitive hosts.

Fasciolosis is a parasitic worm infection caused by the common liver fluke Fasciola hepatica as well as by Fasciola gigantica. The disease is a plant-borne trematode zoonosis, and is classified as a neglected tropical disease (NTD). It affects humans, but its main host is ruminants such as cattle and sheep. The disease progresses through four distinct phases; an initial incubation phase of between a few days up to three months with little or no symptoms; an invasive or acute phase which may manifest with: fever, malaise, abdominal pain, gastrointestinal symptoms, urticaria, anemia, jaundice, and respiratory symptoms. The disease later progresses to a latent phase with less symptoms and ultimately into a chronic or obstructive phase months to years later. In the chronic state the disease causes inflammation of the bile ducts, gall bladder and may cause gall stones as well as fibrosis. While chronic inflammation is connected to increased cancer rates, it is unclear whether fasciolosis is associated with increased cancer risk.

Fasciola hepatica, also known as the common liver fluke or sheep liver fluke, is a parasitic trematode of the class Trematoda, phylum Platyhelminthes. It infects the livers of various mammals, including humans, and is transmitted by sheep and cattle to humans all over the world. The disease caused by the fluke is called fasciolosis or fascioliasis, which is a type of helminthiasis and has been classified as a neglected tropical disease. Fasciolosis is currently classified as a plant/food-borne trematode infection, often acquired through eating the parasite's metacercariae encysted on plants. F. hepatica, which is distributed worldwide, has been known as an important parasite of sheep and cattle for decades and causes significant economic losses in these livestock species, up to £23 million in the UK alone. Because of its relatively large size and economic importance, it has been the subject of many scientific investigations and may be the best-known of any trematode species. F. hepatica's closest relative is Fasciola gigantica. These two flukes are sister species; they share many morphological features and can mate with each other.

Fasciola, commonly known as the liver fluke, is a genus of parasitic trematodes. There are three species within the genus Fasciola: Fasciola nyanzae,Fasciolahepatica and Fasciolagigantica. Fasciola hepatica and F. gigantica are known to form hybrids. Both F. hepatica and F. gigantica and their hybrids infect the liver tissue of a wide variety of mammals, including humans, in a condition known as fascioliasis. F. hepatica measures up to 30 mm by 15 mm, while F. gigantica measures up to 75 mm by 15 mm. Fasciola nyanzae is thought to exclusively infect the common hippopotamus, Hippopotamus amphibius.

Fasciola gigantica is a parasitic flatworm of the class Trematoda, which causes tropical fascioliasis. It is regarded as one of the most important single platyhelminth infections of ruminants in Asia and Africa. The infection is commonly called fasciolosis.

Trematodes are parasitic flatworms of the class Trematoda, specifically parasitic flukes with two suckers: one ventral and the other oral. Trematodes are covered by a tegument, that protects the organism from the environment by providing secretory and absorptive functions.

Paragonimus westermani is the most common species of lung fluke that infects humans, causing paragonimiasis. Human infections are most common in eastern Asia and in South America. Paragonimiasis may present as a sub-acute to chronic inflammatory disease of the lung. It was discovered by Coenraad Kerbert (1849–1927) in 1878.

Echinostoma is a genus of trematodes (flukes), which can infect both humans and other animals. These intestinal flukes have a three-host life cycle with snails or other aquatic organisms as intermediate hosts, and a variety of animals, including humans, as their definitive hosts.

Dicrocoelium dendriticum, the lancet liver fluke, is a parasite fluke that tends to live in cattle or other grazing mammals.

Anthelmintics or antihelminthics are a group of antiparasitic drugs that expel parasitic worms (helminths) and other internal parasites from the body by either stunning or killing them and without causing significant damage to the host. They may also be called vermifuges or vermicides. Anthelmintics are used to treat people who are infected by helminths, a condition called helminthiasis. These drugs are also used to treat infected animals, particularly small ruminants such as goats and sheep.
Moniezia expansa is commonly known as sheep tapeworm or double-pored ruminant tapeworm. It is a large tapeworm inhabiting the small intestines of ruminants such as sheep, goats and cattle. It has been reported from Peru that pigs are also infected. There is an unusual report of human infection in an Egyptian. It is characterized by unarmed scolex, presence of two sets of reproductive systems in each proglottid, and each proglottid being very short but very broad.

Schistosoma spindale is a species of digenetic trematode in the family Schistosomatidae. It causes intestinal schistosomiasis in the ruminants.

Clinostomum marginatum is a species of parasitic fluke. It is commonly called the "Yellow grub". It is found in many freshwater fish in North America, and no fish so far is immune to this parasite. It is also found in frogs. Clinostomum marginatum can also be found in the mouth of aquatic birds such as herons and egrets. They are commonly present in the esophagus of fish-eating birds and reptiles. Eggs of these trematodes are shed in the feces of aquatic birds and released into water. Aquatic birds become hosts of this parasite by ingesting infected freshwater fish. The metacercariae are found right beneath the skin or in the muscles of host fish.
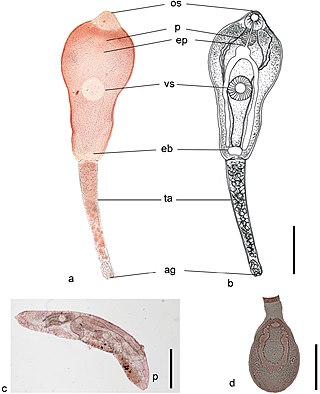
Philophthalmus gralli, commonly known as the Oriental avian eye fluke, parasitises the conjunctival sac of the eyes of many species of birds, including birds of the orders Galliformes and Anseriformes. In Brazil this parasite was reported in native Anseriformes species. It was first discovered by Mathis and Leger in 1910 in domestic chickens from Hanoi, Vietnam. Birds are definitive hosts and freshwater snail species are intermediate hosts. Human cases of philophthalmosis are rare, but have been previously reported in Europe, Asia, and America.

Oxfendazole is a broad spectrum benzimidazole anthelmintic. Its main use is for protecting livestock against roundworm, strongyles and pinworms. Oxfendazole is the sulfoxide metabolite of fenbendazole.

Gastrodiscoides is genus of zoonotic trematode under the class Trematoda. It has only one species, Gastrodiscoides hominis. It is a parasite of a variety of vertebrates, including humans. The first definitive specimen was described from a human subject in 1876. It is prevalent in Bangladesh, India, Burma, China, Kazakhstan, Philippines, Thailand, Vietnam, and the Volga Delta of Russia, with isolated cases from Africa, such as Nigeria. It is especially notable in the Assam, Bengal, Bihar, Madhya Pradesh, Orissa and Uttar Pradesh regions of India.
Paramphistomum cervi, the type species of Paramphistomum, is a parasitic flat worm belonging to the class Trematoda. It is a tiny fluke mostly parasitising livestock ruminants, as well as some wild mammals. Uniquely, unlike most parasites, the adult worms are relatively harmless, but it is the developing juveniles that cause serious disease called paramphistomiasis, especially in cattle and sheep. Its symptoms include profuse diarrhoea, anaemia, lethargy, and often result in death if untreated.
Amphistomiasis is a parasitic disease of livestock animals, more commonly of cattle and sheep, and humans caused by immature helminthic flatworms belonging to the order Echinostomida. The term amphistomiasis is used for broader connotation implying the disease inflicted by members of Echinostomida including the family Paramphistomidae/Gastrodiscidae ; whereas paramphistomiasis is restricted to that of the members of the family Paramphistomidae only. G. discoides and Watsonius watsoni are responsible for the disease in humans, while most paramphistomes are responsible in livestock animals, and some wild mammals. In livestock industry the disease causes heavy economic backlashes due to poor production of milk, meat and wool.

Gastropod-borne parasitic diseases (GPDs) are a group of infectious diseases that require a gastropod species to serve as an intermediate host for a parasitic organism that can infect humans upon ingesting the parasite or coming into contact with contaminated water sources. These diseases can cause a range of symptoms, from mild discomfort to severe, life-threatening conditions, with them being prevalent in many parts of the world, particularly in developing regions. Preventive measures such as proper sanitation and hygiene practices, avoiding contact with infected gastropods and cooking or boiling food properly can help to reduce the risk of these diseases.














