Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2024, there are eight recognized genera. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.

Merkel-cell carcinoma (MCC) is a rare and aggressive skin cancer occurring in about three people per million members of the population. It is also known as cutaneous APUDoma, primary neuroendocrine carcinoma of the skin, primary small cell carcinoma of the skin, and trabecular carcinoma of the skin. Factors involved in the development of MCC include the Merkel cell polyomavirus, a weakened immune system, and exposure to ultraviolet radiation. Merkel-cell carcinoma usually arises on the head, neck, and extremities, as well as in the perianal region and on the eyelid. It is more common in people over sixty years old, Caucasian people, and males. MCC is less common in children.
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.

Yuan Chang is a Taiwanese-born American virologist and pathologist who co-discovered together with her husband, Patrick S. Moore, the Kaposi's sarcoma-associated herpesvirus (KSHV) and Merkel cell polyomavirus, two of the seven known human oncoviruses.

Interferon regulatory factors (IRF) are proteins which regulate transcription of interferons. Interferon regulatory factors contain a conserved N-terminal region of about 120 amino acids, which folds into a structure that binds specifically to the IRF-element (IRF-E) motifs, which is located upstream of the interferon genes. Some viruses have evolved defense mechanisms that regulate and interfere with IRF functions to escape the host immune system. For instance, the remaining parts of the interferon regulatory factor sequence vary depending on the precise function of the protein. The Kaposi sarcoma herpesvirus, KSHV, is a cancer virus that encodes four different IRF-like genes; including vIRF1, which is a transforming oncoprotein that inhibits type 1 interferon activity. In addition, the expression of IRF genes is under epigenetic regulation by promoter DNA methylation.
A transmissible cancer is a cancer cell or cluster of cancer cells that can be transferred between individuals without the involvement of an infectious agent, such as an Oncovirus. The evolution of transmissible cancer has occurred naturally in other animal species, but human cancer transmission is rare. This transfer is typically between members of the same species or closely related species.

OX-2 membrane glycoprotein, also named CD200 is a human protein encoded by the CD200 gene. CD200 gene is in human located on chromosome 3 in proximity to genes encoding other B7 proteins CD80/CD86. In mice CD200 gene is on chromosome 16.
Murid gammaherpesvirus 68 (MuHV-68) is an isolate of the virus species Murid gammaherpesvirus 4, a member of the genus Rhadinovirus. It is a member of the subfamily Gammaherpesvirinae in the family of Herpesviridae. MuHV-68 serves as a model for study of human gammaherpesviruses which cause significant human disease including B-cell lymphoma and Kaposi's sarcoma. The WUMS strain of MuHV-68 was fully sequenced and annotated in 1997, and the necessity of most of its genes in viral replication was characterized by random transposon mutagenesis.
Merkel cell polyomavirus was first described in January 2008 in Pittsburgh, Pennsylvania. It was the first example of a human viral pathogen discovered using unbiased metagenomic next-generation sequencing with a technique called digital transcriptome subtraction. MCV is one of seven currently known human oncoviruses. It is suspected to cause the majority of cases of Merkel cell carcinoma, a rare but aggressive form of skin cancer. Approximately 80% of Merkel cell carcinoma (MCC) tumors have been found to be infected with MCV. MCV appears to be a common—if not universal—infection of older children and adults. It is found in respiratory secretions, suggesting that it might be transmitted via a respiratory route. However, it has also been found elsewhere, such as in shedded healthy skin and gastrointestinal tract tissues, thus its precise mode of transmission remains unknown. In addition, recent studies suggest that this virus may latently infect the human sera and peripheral blood mononuclear cells.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.

Kaposi's sarcoma (KS) is a type of cancer that can form masses in the skin, in lymph nodes, in the mouth, or in other organs. The skin lesions are usually painless, purple and may be flat or raised. Lesions can occur singly, multiply in a limited area, or may be widespread. Depending on the sub-type of disease and level of immune suppression, KS may worsen either gradually or quickly. Except for Classical KS where there is generally no immune suppression, KS is caused by a combination of immune suppression and infection by Human herpesvirus 8.
VG-1 is a B cell line which was derived from primary effusion lymphoma (PEL). It was first established in 2000 by David T. Scadden’s group at Massachusetts General Hospital. It is infected with Kaposi's sarcoma-associated herpesvirus (KSHV), but negative with Epstein–Barr virus (EBV).
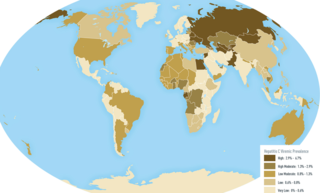
Estimates place the worldwide risk of cancers from infectious causes at 16.1%. Viral infections are risk factors for cervical cancer, 80% of liver cancers, and 15–20% of the other cancers. This proportion varies in different regions of the world from a high of 32.7% in Sub-Saharan Africa to 3.3% in Australia and New Zealand.

Digital transcriptome subtraction (DTS) is a bioinformatics method to detect the presence of novel pathogen transcripts through computational removal of the host sequences. DTS is the direct in silico analogue of the wet-lab approach representational difference analysis (RDA), and is made possible by unbiased high-throughput sequencing and the availability of a high-quality, annotated reference genome of the host. The method specifically examines the etiological agent of infectious diseases and is best known for discovering Merkel cell polyomavirus, the suspect causative agent in Merkel-cell carcinoma.
Large B-cell lymphoma arising in HHV8-associated multicentric Castleman's disease is a type of large B-cell lymphoma, recognized in the WHO 2008 classification. It is sometimes called the plasmablastic form of multicentric Castleman disease. It has sometimes been confused with plasmablastic lymphoma in the literature, although that is a dissimilar specific entity. It has variable CD20 expression and unmutated immunoglobulin variable region genes.
Eva Henriette Gottwein is a virologist and Associate Professor of Microbiology-Immunology at Northwestern University Feinberg School of Medicine in Chicago, Illinois. The main focus of her research is the role of viral miRNAs involved in herpesviral oncogenesis. Gottwein is member of Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Her contributions as a member include the focus on how encoded miRNAs target and function in the human oncogenic herpesvirus Kaposi's sarcoma-associated herpesvirus known as KSHV.

The large tumor antigen is a protein encoded in the genomes of polyomaviruses, which are small double-stranded DNA viruses. LTag is expressed early in the infectious cycle and is essential for viral proliferation. Containing four well-conserved protein domains as well as several intrinsically disordered regions, LTag is a fairly large multifunctional protein; in most polyomaviruses, it ranges from around 600-800 amino acids in length. LTag has two primary functions, both related to replication of the viral genome: it unwinds the virus's DNA to prepare it for replication, and it interacts with proteins in the host cell to dysregulate the cell cycle so that the host's DNA replication machinery can be used to replicate the virus's genome. Some polyomavirus LTag proteins - most notably the well-studied SV40 large tumor antigen from the SV40 virus - are oncoproteins that can induce neoplastic transformation in the host cell.

The small tumor antigen is a protein encoded in the genomes of polyomaviruses, which are small double-stranded DNA viruses. STag is expressed early in the infectious cycle and is usually not essential for viral proliferation, though in most polyomaviruses it does improve replication efficiency. The STag protein is expressed from a gene that overlaps the large tumor antigen (LTag) such that the two proteins share an N-terminal DnaJ-like domain but have distinct C-terminal regions. STag is known to interact with host cell proteins, most notably protein phosphatase 2A (PP2A), and may activate the expression of cellular proteins associated with the cell cycle transition to S phase. In some polyomaviruses - such as the well-studied SV40, which natively infects monkeys - STag is unable to induce neoplastic transformation in the host cell on its own, but its presence may increase the transforming efficiency of LTag. In other polyomaviruses, such as Merkel cell polyomavirus, which causes Merkel cell carcinoma in humans, STag appears to be important for replication and to be an oncoprotein in its own right.













