Related Research Articles

Tumor hypoxia is the situation where tumor cells have been deprived of oxygen. As a tumor grows, it rapidly outgrows its blood supply, leaving portions of the tumor with regions where the oxygen concentration is significantly lower than in healthy tissues. Hypoxic microenvironements in solid tumors are a result of available oxygen being consumed within 70 to 150 μm of tumour vasculature by rapidly proliferating tumor cells thus limiting the amount of oxygen available to diffuse further into the tumor tissue. In order to support continuous growth and proliferation in challenging hypoxic environments, cancer cells are found to alter their metabolism. Furthermore, hypoxia is known to change cell behavior and is associated with extracellular matrix remodeling and increased migratory and metastatic behavior.

Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.
In oncology, the Warburg effect is the observation that most cancer cells release energy predominantly not through the 'usual' citric acid cycle and oxidative phosphorylation in the mitochondria as observed in normal cells, but through a less efficient process of 'aerobic glycolysis' consisting of a high level of glucose uptake and glycolysis followed by lactic acid fermentation taking place in the cytosol, not the mitochondria, even in the presence of abundant oxygen. This observation was first published by Otto Heinrich Warburg, who was awarded the 1931 Nobel Prize in Physiology for his "discovery of the nature and mode of action of the respiratory enzyme". The precise mechanism and therapeutic implications of the Warburg effect, however, remain unclear.

The epidermal growth factor receptor is a transmembrane protein that is a receptor for members of the epidermal growth factor family of extracellular protein ligands.
Double minutes are small fragments of extrachromosomal DNA, which have been observed in a large number of human tumors including breast, lung, ovary, colon, and most notably, neuroblastoma. They are a manifestation of gene amplification as a result of chromothripsis, during the development of tumors, which give the cells selective advantages for growth and survival. This selective advantage is as a result of double minutes frequently harboring amplified oncogenes and genes involved in drug resistance. DMs, like actual chromosomes, are composed of chromatin and replicate in the nucleus of the cell during cell division. Unlike typical chromosomes, they are composed of circular fragments of DNA, up to only a few million base pairs in size, and contain no centromere or telomere. Further to this, they often lack key regulatory elements, allowing genes to be constitutively expressed. The term ecDNA may be used to refer to DMs in a more general manner.

Receptor tyrosine-protein kinase erbB-2 is a protein that normally resides in the membranes of cells and is encoded by the ERBB2 gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently referred to as HER2 or CD340.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
Extrachromosomal DNA is any DNA that is found off the chromosomes, either inside or outside the nucleus of a cell. Most DNA in an individual genome is found in chromosomes contained in the nucleus. Multiple forms of extrachromosomal DNA exist, and, while some of these serve important biological functions, they can also play a role in diseases such as cancer.
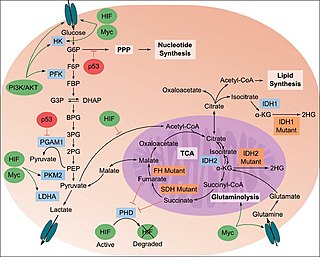
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.

The Warburg hypothesis, sometimes known as the Warburg theory of cancer, postulates that the driver of tumorigenesis is an insufficient cellular respiration caused by insult to mitochondria. The term Warburg effect in oncology describes the observation that cancer cells, and many cells grown in vitro, exhibit glucose fermentation even when enough oxygen is present to properly respire. In other words, instead of fully respiring in the presence of adequate oxygen, cancer cells ferment. The Warburg hypothesis was that the Warburg effect was the root cause of cancer. The current popular opinion is that cancer cells ferment glucose while keeping up the same level of respiration that was present before the process of carcinogenesis, and thus the Warburg effect would be defined as the observation that cancer cells exhibit glycolysis with lactate production and mitochondrial respiration even in the presence of oxygen.

KRAS is a gene that provides instructions for making a protein called K-Ras, a part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). It is called KRAS because it was first identified as a viral oncogene in the KirstenRAt Sarcoma virus. The oncogene identified was derived from a cellular genome, so KRAS, when found in a cellular genome, is called a proto-oncogene.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

Proto-oncogene tyrosine-protein kinase ROS is an enzyme that in humans is encoded by the ROS1 gene.

Rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) is a protein that in humans is encoded by the RICTOR gene.

Lysosomal-associated transmembrane protein 4B is a protein that in humans is encoded by the LAPTM4B gene.

Neratinib (INN), sold under the brand name Nerlynx, is a tyrosine kinase inhibitor anti-cancer medication used for the treatment of breast cancer.
mTOR Complex 2 (mTORC2) is an acutely rapamycin-insensitive protein complex formed by serine/threonine kinase mTOR that regulates cell proliferation and survival, cell migration and cytoskeletal remodeling. The complex itself is rather large, consisting of seven protein subunits. The catalytic mTOR subunit, DEP domain containing mTOR-interacting protein (DEPTOR), mammalian lethal with sec-13 protein 8, and TTI1/TEL2 complex are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with rictor 1 and 2 (Protor1/2) can only be found in mTORC2. Rictor has been shown to be the scaffold protein for substrate binding to mTORC2.
Antineoplastic resistance, often used interchangeably with chemotherapy resistance, is the resistance of neoplastic (cancerous) cells, or the ability of cancer cells to survive and grow despite anti-cancer therapies. In some cases, cancers can evolve resistance to multiple drugs, called multiple drug resistance.
Extrachromosomal circular DNA (eccDNA) is a type of double-stranded circular DNA structure that was first discovered in 1964 by Alix Bassel and Yasuo Hotta. In contrast to previously identified circular DNA structures, eccDNA are circular DNA found in the eukaryotic nuclei of plant and animal cells. Extrachromosomal circular DNA is derived from chromosomal DNA, can range in size from 50 base pairs to several mega-base pairs in length, and can encode regulatory elements and full-length genes. eccDNA has been observed in various eukaryotic species and it is proposed to be a byproduct of programmed DNA recombination events, such as V(D)J recombination.
References
- ↑ "Team". Paul Mischel Lab. Retrieved 2021-04-15.
- ↑ "Paul Salomon Mischel's Profile | Stanford Profiles". profiles.stanford.edu. Retrieved 2021-04-15.
- ↑ "Paul Mischel | ChEM-H". chemh.stanford.edu. Retrieved 2021-04-15.
- 1 2 "The American Society for Clinical Investigation" . Retrieved 2019-01-31.
- 1 2 "Ludwig San Diego's Paul Mischel elected AAAS Fellow". EurekAlert!. Retrieved 2019-01-29.
- 1 2 "2015 AAAS Fellows Recognized for Contributions to Advancing Science". American Association for the Advancement of Science. Retrieved 2019-01-29.
- 1 2 "Dr. Paul Mischel, Pathologist in La Jolla, CA | US News Doctors".
- ↑ "Faculty Database Production Server | David Geffen School of Medicine at UCLA". people.healthsciences.ucla.edu. Retrieved 2019-01-30.
- ↑ "Paul Mischel Lab - University of California, San Diego". Paul Mischel Lab. Retrieved 2019-06-13.
- ↑ "Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer". cgap.nci.nih.gov. Retrieved 2018-11-27.
- ↑ Nathanson, David A.; Gini, Beatrice; Mottahedeh, Jack; Visnyei, Koppany; Koga, Tomoyuki; Gomez, German; Eskin, Ascia; Hwang, Kiwook; Mischel, Paul S. (2014-01-03). "Targeted Therapy Resistance Mediated by Dynamic Regulation of Extrachromosomal Mutant EGFR DNA". Science. 343 (6166): 72–76. Bibcode:2014Sci...343...72N. doi:10.1126/science.1241328. ISSN 0036-8075. PMC 4049335 . PMID 24310612.
- ↑ Turner, Kristen M.; Deshpande, Viraj; Beyter, Doruk; Koga, Tomoyuki; Rusert, Jessica; Lee, Catherine; Li, Bin; Arden, Karen; Mischel, Paul S. (2017-02-08). "Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity". Nature. 543 (7643): 122–125. Bibcode:2017Natur.543..122T. doi:10.1038/nature21356. ISSN 0028-0836. PMC 5334176 . PMID 28178237.
- ↑ Fikes, Bradley J. (9 February 2017). "Cancer genes hide outside chromosomes". sandiegouniontribune.com. Retrieved 2019-01-31.
- ↑ "Non-Chromosomal DNA Drives Tumor Evolution". The Scientist Magazine®. Retrieved 2019-02-01.
- ↑ Wu, Sihan; Turner, Kristen M.; Nguyen, Nam; Raviram, Ramya; Erb, Marcella; Santini, Jennifer; Luebeck, Jens; Rajkumar, Utkrisht; Diao, Yarui; Li, Bin; Zhang, Wenjing (November 2019). "Circular ecDNA promotes accessible chromatin and high oncogene expression". Nature. 575 (7784): 699–703. Bibcode:2019Natur.575..699W. doi:10.1038/s41586-019-1763-5. ISSN 0028-0836. PMC 7094777 . PMID 31748743.
- ↑ Furnari, Frank B.; Cloughesy, Timothy F.; Cavenee, Webster K.; Mischel, Paul S. (2015-04-09). "Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma". Nature Reviews Cancer. 15 (5): 302–310. doi:10.1038/nrc3918. ISSN 1474-175X. PMC 4875778 . PMID 25855404.
- ↑ Verhaak, Roel G. W.; Bafna, Vineet; Mischel, Paul S. (May 2019). "Extrachromosomal oncogene amplification in tumour pathogenesis and evolution". Nature Reviews Cancer. 19 (5): 283–288. doi:10.1038/s41568-019-0128-6. ISSN 1474-175X. PMC 7168519 . PMID 30872802.
- ↑ Pennisi, Elizabeth (2017-06-09). "Circular DNA throws biologists for a loop". Science. 356 (6342): 996. Bibcode:2017Sci...356..996P. doi:10.1126/science.356.6342.996. ISSN 0036-8075. PMID 28596318.
- ↑ Aranda, Victoria (2014-01-07). "Cancer: Extrachromosomal resistance". Nature Medicine. 20: 28. doi:10.1038/nm.3452. ISSN 1546-170X. S2CID 45444065.
- ↑ Zimmer, Carl (2019-11-20). "Scientists Are Just Beginning to Understand Mysterious DNA Circles Common in Cancer Cells". The New York Times. ISSN 0362-4331 . Retrieved 2020-02-05.
- ↑ Masui, Kenta; Tanaka, Kazuhiro; Akhavan, David; Babic, Ivan; Gini, Beatrice; Matsutani, Tomoo; Iwanami, Akio; Liu, Feng; Mischel, Paul S. (2013-11-05). "mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc". Cell Metabolism. 18 (5): 726–739. doi:10.1016/j.cmet.2013.09.013. ISSN 1932-7420. PMC 3840163 . PMID 24140020.
- ↑ Masui, Kenta; Tanaka, Kazuhiro; Ikegami, Shiro; Villa, Genaro R.; Yang, Huijun; Yong, William H.; Cloughesy, Timothy F.; Yamagata, Kanato; Mischel, Paul S. (2015-07-28). "Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance". Proceedings of the National Academy of Sciences. 112 (30): 9406–9411. Bibcode:2015PNAS..112.9406M. doi: 10.1073/pnas.1511759112 . ISSN 0027-8424. PMC 4522814 . PMID 26170313.
- ↑ Babic, Ivan; Anderson, Erik S.; Tanaka, Kazuhiro; Guo, Deliang; Masui, Kenta; Li, Bing; Zhu, Shaojun; Gu, Yuchao; Mishcel, Paul S. (2013-06-04). "EGFR mutation-induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer". Cell Metabolism. 17 (6): 1000–1008. doi:10.1016/j.cmet.2013.04.013. ISSN 1932-7420. PMC 3679227 . PMID 23707073.
- ↑ Gu, Yuchao; Albuquerque, Claudio P.; Braas, Daniel; Zhang, Wei; Villa, Genaro R.; Bi, Junfeng; Ikegami, Shiro; Masui, Kenta; Mischel, Paul S. (2017-07-06). "mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT". Molecular Cell. 67 (1): 128–138.e7. doi:10.1016/j.molcel.2017.05.030. ISSN 1097-4164. PMC 5521991 . PMID 28648777.
- ↑ Guo, Deliang; Reinitz, Felicia; Youssef, Mary; Hong, Cynthia; Nathanson, David; Akhavan, David; Kuga, Daisuke; Amzajerdi, Ali Nael; Mischel, Paul S. (2011-10-01). "An LXR Agonist Promotes Glioblastoma Cell Death through Inhibition of an EGFR/AKT/SREBP-1/LDLR–Dependent Pathway". Cancer Discovery. 1 (5): 442–456. doi:10.1158/2159-8290.CD-11-0102. ISSN 2159-8274. PMC 3207317 . PMID 22059152.
- ↑ Chowdhry, Sudhir; Zanca, Ciro; Rajkumar, Utkrisht; Koga, Tomoyuki; Diao, Yarui; Raviram, Ramya; Liu, Feng; Turner, Kristen; Yang, Huijun; Brunk, Elizabeth; Bi, Junfeng (May 2019). "NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling". Nature. 569 (7757): 570–575. Bibcode:2019Natur.569..570C. doi:10.1038/s41586-019-1150-2. ISSN 0028-0836. PMC 7138021 . PMID 31019297.
- ↑ Bi, Junfeng; Ichu, Taka-Aki; Zanca, Ciro; Yang, Huijun; Zhang, Wei; Gu, Yuchao; Chowdhry, Sudhir; Reed, Alex; Ikegami, Shiro; Turner, Kristen M.; Zhang, Wenjing (September 2019). "Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling". Cell Metabolism. 30 (3): 525–538.e8. doi:10.1016/j.cmet.2019.06.014. PMC 6742496 . PMID 31303424.
- ↑ Masui, Kenta; Cavenee, Webster K.; Mischel, Paul S. (2014-07-25). "mTORC2 in the center of cancer metabolic reprogramming". Trends in Endocrinology and Metabolism. 25 (7): 364–373. doi:10.1016/j.tem.2014.04.002. ISSN 1879-3061. PMC 4077930 . PMID 24856037.
- ↑ Wu, Si-Han; Bi, Jun-Feng; Cloughesy, Timothy; Cavenee, Webster K.; Mischel, Paul S. (2014). "Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance". Cancer Biology & Medicine. 11 (4): 255–263. doi:10.7497/j.issn.2095-3941.2014.04.004. ISSN 2095-3941. PMC 4296088 . PMID 25610711.
- ↑ Bi, Junfeng; Wu, Sihan; Zhang, Wenjing; Mischel, Paul S. (2018-05-23). "Targeting cancer's metabolic co-dependencies: A landscape shaped by genotype and tissue context". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1870 (1): 76–87. doi:10.1016/j.bbcan.2018.05.002. ISSN 1879-2561. PMC 6193564 . PMID 29775654.
- ↑ Bi, Junfeng; Chowdhry, Sudhir; Wu, Sihan; Zhang, Wenjing; Masui, Kenta; Mischel, Paul S. (January 2020). "Altered cellular metabolism in gliomas — an emerging landscape of actionable co-dependency targets". Nature Reviews Cancer. 20 (1): 57–70. doi:10.1038/s41568-019-0226-5. ISSN 1474-1768. PMID 31806884. S2CID 208768689.
- ↑ "SNO Awards". www.soc-neuro-onc.org. Retrieved 2019-01-31.
- ↑ Williams, Ruth (2008-06-30). "Paul Mischel: All about brains". The Journal of Cell Biology. 181 (7): 1044–1045. doi:10.1083/jcb.1817pi. ISSN 0021-9525. PMC 2442209 . PMID 18591424.