Related Research Articles

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.
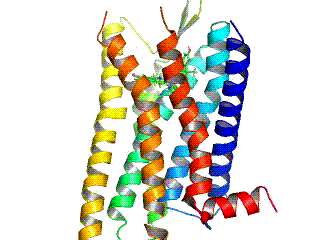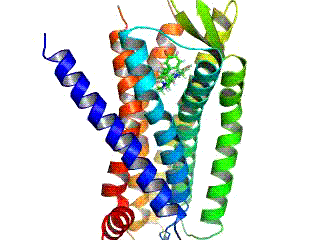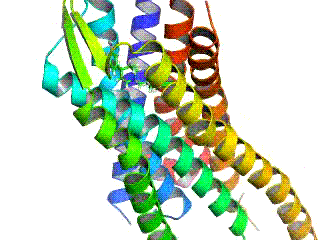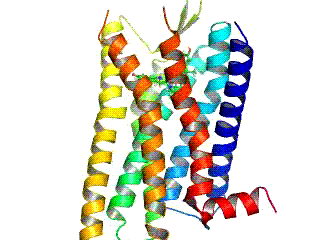
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.

Chemokines, or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses, chemokines are important for biological processes, including morphogenesis and wound healing, as well as in the pathogenesis of diseases like cancers.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the mu opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.
Protease-activated receptors (PAR) are a subfamily of related G protein-coupled receptors that are activated by cleavage of part of their extracellular domain. They are highly expressed in platelets, and also on endothelial cells, myocytes and neurons.

C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 is a protein that in humans is encoded by the CXCR4 gene. The protein is a CXC chemokine receptor.

The Urokinase receptor, also known as urokinase plasminogen activator surface receptor (uPAR) or CD87, is a protein encoded in humans by the PLAUR gene. It is a multidomain glycoprotein tethered to the cell membrane with a glycosylphosphotidylinositol (GPI) anchor. uPAR was originally identified as a saturable binding site for urokinase on the cell surface.
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.

G protein-coupled receptor kinases are a family of protein kinases within the AGC group of kinases. Like all AGC kinases, GRKs use ATP to add phosphate to Serine and Threonine residues in specific locations of target proteins. In particular, GRKs phosphorylate intracellular domains of G protein-coupled receptors (GPCRs). GRKs function in tandem with arrestin proteins to regulate the sensitivity of GPCRs for stimulating downstream heterotrimeric G protein and G protein-independent signaling pathways.

Plerixafor is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients into the bloodstream. The stem cells are then extracted from the blood and transplanted back to the patient. The drug was developed by AnorMED, which was subsequently bought by Genzyme.

Growth hormone secretagogue receptor(GHS-R), also known as ghrelin receptor, is a G protein-coupled receptor that binds growth hormone secretagogues (GHSs), such as ghrelin, the "hunger hormone". The role of GHS-R is thought to be in regulating energy homeostasis and body weight. In the brain, they are most highly expressed in the hypothalamus, specifically the ventromedial nucleus and arcuate nucleus. GSH-Rs are also expressed in other areas of the brain, including the ventral tegmental area, hippocampus, and substantia nigra. Outside the central nervous system, too, GSH-Rs are also found in the liver, in skeletal muscle, and even in the heart.

G protein-coupled estrogen receptor 1 (GPER), also known as G protein-coupled receptor 30 (GPR30), is a protein that in humans is encoded by the GPER gene. GPER binds to and is activated by the female sex hormone estradiol and is responsible for some of the rapid effects that estradiol has on cells.

Interleukin 8 receptor, beta is a chemokine receptor. IL8RB is also known as CXCR2, and CXCR2 is now the IUPHAR Committee on Receptor Nomenclature and Drug classification-recommended name.

Interleukin 8 receptor, alpha is a chemokine receptor. This name and the corresponding gene symbol IL8RA have been replaced by the HGNC approved name C-X-C motif chemokine receptor 1 and the approved symbol CXCR1. It has also been designated as CD181. The IUPHAR Committee on Receptor Nomenclature and Drug Classification use the HGNC recommended name, CXCR1.

Proteinase-activated receptor 1 (PAR1) also known as Protease-activated receptor 1 or coagulation factor II (thrombin) receptor is a protein that in humans is encoded by the F2R gene. PAR1 is a G protein-coupled receptor and one of four protease-activated receptors involved in the regulation of thrombotic response. Highly expressed in platelets and endothelial cells, PAR1 plays a key role in mediating the interplay between coagulation and inflammation, which is important in the pathogenesis of inflammatory and fibrotic lung diseases. It is also involved both in disruption and maintenance of endothelial barrier integrity, through interaction with either thrombin or activated protein C, respectively.

Protease-activated receptor 4 (PAR-4), also known as coagulation factor II (thrombin) receptor-like 3, is a protein that in humans is encoded by the F2RL3 gene.

G protein-coupled receptor 56 also known as TM7XN1 is a protein encoded by the ADGRG1 gene. GPR56 is a member of the adhesion GPCR family. Adhesion GPCRs are characterized by an extended extracellular region often possessing N-terminal protein modules that is linked to a TM7 region via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.

A GPCR oligomer is a protein complex that consists of a small number of G protein-coupled receptors (GPCRs). It is held together by covalent bonds or by intermolecular forces. The subunits within this complex are called protomers, while unconnected receptors are called monomers. Receptor homomers consist of identical protomers, while heteromers consist of different protomers.
References
- ↑ Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A (January 2002). "Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides". Proc. Natl. Acad. Sci. U.S.A. 99 (2): 643–8. doi: 10.1073/pnas.022460899 . PMC 117359 . PMID 11805322.
- 1 2 Kuliopulos A, Covic L (December 2003). "Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis". Life Sci. 74 (2–3): 255–62. doi:10.1016/j.lfs.2003.09.012. PMID 14607253.
- ↑ Kubo S, Ishiki T, Doe I, et al. (December 2006). "Distinct activity of peptide mimetic intracellular ligands (pepducins) for proteinase-activated receptor-1 in multiple cells/tissues". Ann. N. Y. Acad. Sci. 1091: 445–59. doi:10.1196/annals.1378.087. PMID 17341635. S2CID 24166439.
- ↑ "Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells". 2010. Archived from the original on 2011-07-24. Retrieved 2011-03-02.
- ↑ Zhang, P.; Gruber, A.; Kasuda, S.; Kimmelstiel, C.; O’Callaghan, K.; Bohm, A.; Baleja, J.D.; Covic, L. & Kuliopulos, A. (2012). "Suppression of Arterial Thrombosis with Affecting Hemostatic Parameters with a Cell-Penetrating PAR1 Pepducin". Circulation. 126 (1): 83–91. doi:10.1161/circulationaha.112.091918. PMC 3423084 . PMID 22705889.
- ↑ Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A (2011). "Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models". Cell-Penetrating Peptides. Methods in Molecular Biology. Vol. 683. Clifton, N.J. pp. 259–75. doi:10.1007/978-1-60761-919-2_19. ISBN 978-1-60761-918-5. PMC 3780409 . PMID 21053136.
- ↑ O’Callaghan, K.; Kuliopulos, A.; Covic, L. (2012). "Turning Receptors On and Off with Intracellular Pepducins: New Insights into G-protein Coupled Receptor Drug Development". J Biol Chem. 287 (16): 12787–96. doi: 10.1074/jbc.r112.355461 . PMC 3339939 . PMID 22374997.