Pro-opiomelanocortin (POMC) is a precursor polypeptide with 241 amino acid residues. POMC is synthesized in corticotrophs of the anterior pituitary from the 267-amino-acid-long polypeptide precursor pre-pro-opiomelanocortin (pre-POMC), by the removal of a 26-amino-acid-long signal peptide sequence during translation. POMC is part of the central melanocortin system.
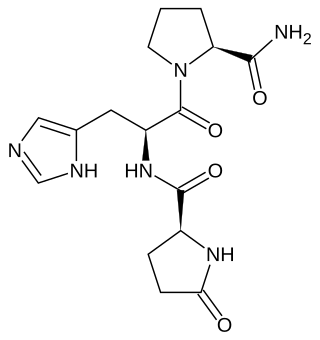
Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.

Steroid 21-hydroxylase is a protein that in humans is encoded by the CYP21A2 gene. The protein is an enzyme that hydroxylates steroids at the C21 position on the molecule. Naming conventions for enzymes are based on the substrate acted upon and the chemical process performed. Biochemically, this enzyme is involved in the biosynthesis of the adrenal gland hormones aldosterone and cortisol, which are important in blood pressure regulation, sodium homeostasis and blood sugar control. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, within metabolic pathways which in humans ultimately lead to aldosterone and cortisol creation—deficiency in the enzyme may cause congenital adrenal hyperplasia.

The adrenocorticotropic hormone receptor or ACTH receptor also known as the melanocortin receptor 2 or MC2 receptor is a type of melanocortin receptor (type 2) which is specific for ACTH. A G protein–coupled receptor located on the external cell plasma membrane, it is coupled to Gαs and upregulates levels of cAMP by activating adenylyl cyclase. The ACTH receptor plays a role in immune function and glucose metabolism.

Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine.
In enzymology, a peptidylglycine monooxygenase (EC 1.14.17.3) is an enzyme that catalyzes the chemical reaction

Corticotropin-releasing hormone receptor 2 (CRHR2) is a protein, also known by the IUPHAR-recommended name CRF2, that is encoded by the CRHR2 gene and occurs on the surfaces of some mammalian cells. CRF2 receptors are type 2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH, a peptide of 41 amino acids synthesized in the hypothalamus, is the principal neuroregulator of the hypothalamic-pituitary-adrenal axis, signaling via guanine nucleotide-binding proteins (G proteins) and downstream effectors such as adenylate cyclase. The CRF2 receptor is a multi-pass membrane protein with a transmembrane domain composed of seven helices arranged in a V-shape. CRF2 receptors are activated by two structurally similar peptides, urocortin II, and urocortin III, as well as CRH.

Shekel Somatostatin receptor type 3 is a protein that in humans is encoded by the SSTR3 gene.

cAMP-dependent protein kinase type I-alpha regulatory subunit is an enzyme that in humans is encoded by the PRKAR1A gene.

In enzymology, an amidase (EC 3.5.1.4, acylamidase, acylase (misleading), amidohydrolase (ambiguous), deaminase (ambiguous), fatty acylamidase, N-acetylaminohydrolase (ambiguous)) is an enzyme that catalyzes the hydrolysis of an amide. In this way, the two substrates of this enzyme are an amide and H2O, whereas its two products are monocarboxylate and NH3.

Kalirin, also known as Huntingtin-associated protein-interacting protein (HAPIP), protein duo (DUO), or serine/threonine-protein kinase with Dbl- and pleckstrin homology domain, is a protein that in humans is encoded by the KALRN gene. Kalirin was first identified in 1997 as a protein interacting with huntingtin-associated protein 1. Is also known to play an important role in nerve growth and axonal development.

Luteinizing hormone subunit beta also known as lutropin subunit beta or LHβ is a polypeptide that in association with an alpha subunit common to all gonadotropin hormones forms the reproductive signaling molecule luteinizing hormone. In humans it is encoded by the LHB gene.

U2AF homology motif (UHM) kinase 1, also known as UHMK1, is a protein which in humans is encoded by the UHMK1 gene.
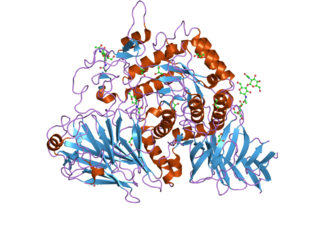
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the MGAM gene.
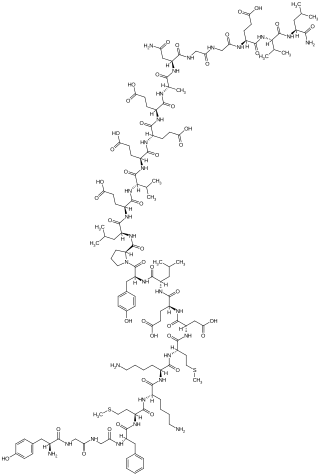
Amidorphin is an endogenous, C-terminally amidated, opioid peptide generated as a cleavage product of proenkephalin A in some mammalian species; in humans and most other species, the peptide is 1 residue longer and is not amidated. Amidorphin is widely distributed in the mammalian brain, with particularly high concentrations found in the striatum, and outside of the brain in adrenal medulla and posterior pituitary. The 26-residue peptide named amidorphin is found in several species including bovine, sheep, and pig. Humans and commonly studied lab animals produce a 27-residue peptide that does not have an amidated C-terminal residue; this is due to the absence of a Gly in the precursor sequence and replacement with Ala, which is not a substrate for the amidating enzyme. The properties of the 27-residue peptide are presumably similar to those of amidorphin, although this has not been adequately tested.

DBH-like monooxygenase protein 1, also known as monooxygenase X, is an enzyme that in humans is encoded by the MOXD1 gene.

Ras association domain-containing protein 9 (RASSF9), also known as PAM COOH-terminal interactor protein 1 (PCIP1) or peptidylglycine alpha-amidating monooxygenase COOH-terminal interactor (PAMCI) is a protein that in humans is encoded by the RASSF9 gene.
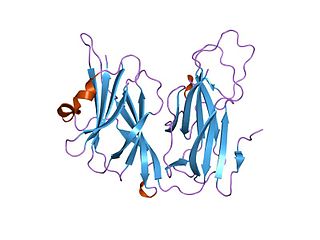
In molecular biology, the copper type II ascorbate-dependent monooxygenases are a class of enzymes that require copper as a cofactor and which use ascorbate as an electron donor. This family contains two related enzymes, dopamine beta-monooxygenase EC 1.14.17.1 and peptidylglycine alpha-amidating monooxygenase EC 1.14.17.3. There are a few regions of sequence similarities between these two enzymes, two of these regions contain clusters of conserved histidine residues which are most probably involved in binding copper.