
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Acesulfame potassium, also known as acesulfame K or Ace K, is a synthetic calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

Perilla frutescensvar.crispa, also known by its Japanese name shiso, is a cultigen of Perilla frutescens, a herb in the mint family Lamiaceae. It is native to the mountainous regions of China and India, but is now found worldwide. The plant occurs in several forms, as defined by the characteristics of their leaves, including red, green, bicolor, and ruffled. Shiso is perennial and may be cultivated as an annual in temperate climates. Different parts of the plant are used in East Asian and Southeast Asian cuisine.

Monellin, a sweet protein, was discovered in 1969 in the fruit of the West African shrub known as serendipity berry ; it was first reported as a carbohydrate. The protein was named in 1972 after the Monell Chemical Senses Center in Philadelphia, U.S.A., where it was isolated and characterized.

Lactisole is the sodium salt and commonly supplied form of 2-(4-methoxyphenoxy)propionic acid, a natural carboxylic acid found in roasted coffee beans. Like gymnemic acid, it has the property of masking sweet flavors and is used for this purpose in the food industry.

Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketones, and sugar alcohols. Some are sweet at very low concentrations, allowing their use as non-caloric sugar substitutes. Such non-sugar sweeteners include saccharin and aspartame. Other compounds, such as miraculin, may alter perception of sweetness itself.

Perilla oil is an edible vegetable oil derived from perilla seeds. Having a distinct nutty aroma and taste, the oil pressed from the toasted perilla seeds is used as a flavor enhancer, condiment, and cooking oil in Korean cuisine. The oil pressed from untoasted perilla seeds is used for non-culinary purposes.

Perillaldehyde, perillic aldehyde or perilla aldehyde, is a natural organic compound found most abundantly in the annual herb perilla, but also in a wide variety of other plants and essential oils. It is a monoterpenoid containing an aldehyde functional group.
Maltisorb is a natural sweetening alcohol made by Roquette of France. It can be used in baking, especially in making fillings for cakes and cookies. It is available in a number of grades, labelled M59, M200 and M40. Maltisorb is a brand name for crystalline maltitol, the formula for which Roquette licenses from Towa Chemical Industry of Japan.

Hovenia dulcis, the Japanese raisin tree or oriental raisin tree, is a hardy tree found in Asia, from Eastern China and Korea to the Himalayas, growing preferably in a sunny position on moist sandy or loamy soils. The tree known for its health benefits when consumed in tea, introduced as an ornamental tree to several countries, also bears edible fruit. It is considered to be one of the most pervasive invaders in Brazilian subtropical forests.
Gymnemic acids are a class of chemical compounds isolated from the leaves of Gymnema sylvestre (Asclepiadaceae). They are anti-sweet compounds, or sweetness inhibitors. After chewing the leaves, solutions sweetened with sugar taste like water.
Hodulcine are glycosides which are isolated from the leaves of Hovenia dulcis Thunb. (Rhamnaceae) also known as Japanese Raisin Tree.
Several glycosides homologue have been found in this plant and although hoduloside 1 exhibits the highest anti-sweet activity, it is less potent than gymnemic acid 1.
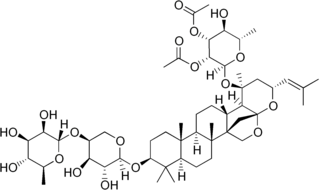
Ziziphin, a triterpene glycoside which exhibits taste-modifying properties, has been isolated from the leaves of Ziziphus jujuba (Rhamnaceae).

Jangajji (장아찌) or pickled vegetables is a type of banchan made by pickling vegetables. Unlike kimchi, jangajji is non-fermented vegetables, usually pickled in soy sauce, soybean paste, or chili paste. Jangajji dishes are usually preserved for a long period of time, and served with a drizzle of sesame oil. Preserved foods like jangajji were developed to attain a certain level of vegetable consumption during the long, harsh winters on the Korean peninsula.
Suosan is calorie-free artificial sweetener derived from β-alanine, discovered in 1948 by Petersen et Muller.

Osladine is a high-intensity sweetener isolated from the rhizome of Polypodium vulgare. It is a saponin, sapogenin steroid glycoside, 500 times sweeter than sucrose.
Perilla may refer to any of the following subjects:

Perilla is a genus consisting of one major Asiatic crop species Perilla frutescens and a few wild species in nature belonging to the mint family, Lamiaceae. The genus encompasses several distinct varieties of Asian herb, seed, and vegetable crop, including P. frutescens (deulkkae) and P. frutescens var. crispa (shiso). The genus name Perilla is also a frequently employed common name ("perilla"), applicable to all varieties. Perilla varieties are cross-fertile and intra-specific hybridization occurs naturally. Some varieties are considered invasive.
Atlas Powder Company was an American explosives and chemicals company. It was one of the two companies that emerged out of a court-ordered breakup of the explosives monopoly of Du Pont Powder Company, the explosives and gunpowder company founded by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours.
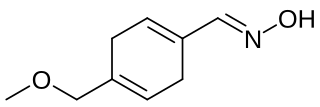
Oxime V is a chemical compound that has been studied as a potential sweetener. Oxime V was first reported in 1976 as a synthetic analog of the artificial sweetener perillartine. It is about 450 times as sweet as sucrose and is more water-soluble than perillartine. Its metabolism and toxicology have been investigated, and it has been found to have promising properties, but it is not currently marketed.















