
Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words smoke and fog to refer to smoky fog due to its opacity, and odor. The word was then intended to refer to what was sometimes known as pea soup fog, a familiar and serious problem in London from the 19th century to the mid-20th century, where it was commonly known as a London particular or London fog. This kind of visible air pollution is composed of nitrogen oxides, sulfur oxide, ozone, smoke and other particulates. Man-made smog is derived from coal combustion emissions, vehicular emissions, industrial emissions, forest and agricultural fires and photochemical reactions of these emissions.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
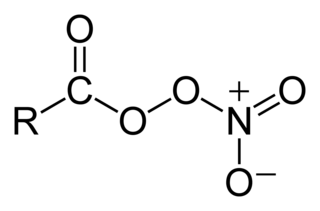
In organic chemistry, peroxyacyl nitrates are powerful respiratory and eye irritants present in photochemical smog. They are nitrates produced in the thermal equilibrium between organic peroxy radicals by the gas-phase oxidation of a variety of volatile organic compounds (VOCs), or by aldehydes and other oxygenated VOCs oxidizing in the presence of NO2.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

The hydroperoxyl radical, also known as the hydrogen superoxide, is the protonated form of superoxide with the chemical formula HO2, also written HOO•. This species plays an important role in the atmosphere and as a reactive oxygen species in cell biology.
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule.
Photo-catalytic concrete is a formulation of concrete used as pavers and other structural concrete that includes titanium dioxide (TiO2) as an admixture or superficial layer. Titanium dioxide is a heterogeneous photocatalyst that uses sunlight and moisture to absorb and render oxides of nitrogen (NO and NO2) into nitrate ions (NO3−), which are then either washed away by rain or soaked into the concrete to form stable compounds.

Tropospheric ozone depletion events are phenomena that reduce the concentration of ozone in the earth's troposphere. Ozone (O3) is a trace gas which has been of concern because of its unique dual role in different layers of the lower atmosphere. Apart from absorbing UV-B radiation and converting solar energy into heat in the stratosphere, ozone in the troposphere provides greenhouse effect and controls the oxidation capacity of the atmosphere.

In chemistry, a photoinitiator is a molecule that creates reactive species when exposed to radiation. Synthetic photoinitiators are key components in photopolymers.

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
Paul O. Wennberg is the R. Stanton Avery Professor of Atmospheric Chemistry and Environmental Science and Engineering at the California Institute of Technology (Caltech). He is the director of the Ronald and Maxine Linde Center for Global Environmental Science. He is chair of the Total Carbon Column Observing Network and a founding member of the Orbiting Carbon Observatory project, which created NASA's first spacecraft for analysis of carbon dioxide in the atmosphere. He is also the principal investigator for the Mars Atmospheric Trace Molecule Occultation Spectrometer (MATMOS) to investigate trace gases in Mars's atmosphere.
Sulfur mononitride is an inorganic compound with the molecular formula SN. It is the sulfur analogue of and isoelectronic to the radical nitric oxide, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
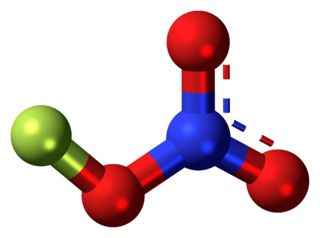
Fluorine nitrate is an unstable derivative of nitric acid with the formula FNO
3. It is shock-sensitive. Due to its instability, it is often produced from chlorine nitrate as needed. Fluorine Nitrate is an inert molecule thought to play a significant role in atmospheric chemistry.
In atmospheric chemistry, the Leighton relationship is an equation that determines the concentration of tropospheric ozone in areas polluted by the presence of nitrogen oxides. Ozone in the troposphere is primarily produced through the photolysis of nitrogen dioxide by photons with wavelengths (λ) less than 420 nanometers, which are able to reach the lowest levels of the atmosphere, through the following mechanism:
Barbara J. Finlayson-Pitts is a Canadian-American atmospheric chemist. She is a professor in the chemistry department at the University of California, Irvine and is the Director of AirUCI Institute. Finlayson-Pitts and James N. Pitts, Jr. are the authors of Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (1999). She has been a member of the National Academy of Sciences since 2006 and is the laureate for the 2017 Garvan–Olin Medal. In 2016 she co-chaired the National Academy of Science report "The Future of Atmospheric Chemistry Research"
Jennifer Logan is an atmospheric scientist known for her research on how human activities influence the atmosphere, particularly with respect to biomass burning and the ozone hole.

John W. Birks is an American atmospheric chemist and entrepreneur who is best known for co-discovery with Paul Crutzen of the potential atmospheric effects of nuclear war known as nuclear winter. His most recent awards include the 2019 Haagen-Smit Clean Air Award for his contributions to atmospheric chemistry and the 2022 Future of Life Award for discovery of the nuclear winter effect.












