Examples
- 1(1,3)-benzenacycloheptaphane
- 1,4(1,4)-dibenzenacyclohexaphane (right hand side)
Phanes are abstractions of highly complex organic molecules introduced for simplification of the naming of these highly complex molecules.
Systematic nomenclature of organic chemistry consists of building a name for the structure of an organic compound by a collection of names of its composite parts but describing also its relative positions within the structure. Naming information is summarised by IUPAC: [1] [2] [3]
"Phane nomenclature is a new method for building names for organic structures by assembling names that describe component parts of a complex structure. It is based on the idea that a relatively simple skeleton for a parent hydride can be modified by an operation called 'amplification', a process that replaces one or more special atoms (superatoms) of a simplified skeleton by multiatomic structures".
Whilst the cyclophane name describes only a limited number of sub-structures of benzene rings interconnected by individual atoms or chains, 'phane' is a class name which includes others, hence heterocyclic rings as well. Therefore, the various cyclophanes are perfectly good for the general class of phanes as well keeping in mind that the cyclic structures in phanes could have much greater diversity.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
A substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.
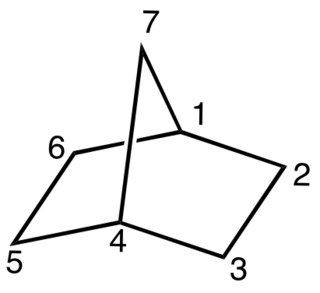
In chemistry, a bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.
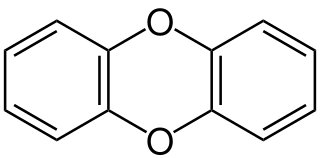
Dibenzo-1,4-dioxin, also dibenzodioxin or dibenzo-p-dioxin (dibenzo-para-dioxin), is a polycyclic heterocyclic organic compound in which two benzene rings are connected by a 1,4-dioxin ring. Its molecular formula is C12H8O2. The two oxygen atoms occupy opposite (para-) positions in the six-membered dioxin ring.
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.

Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.
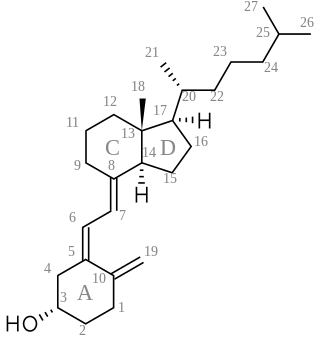
A secosteroid is a type of steroid with a "broken" ring. The word secosteroid derives from the Latin verb secare meaning "to cut", and 'steroid'. Secosteroids are alternatively described as a subclass of steroids or derived from steroids.

Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane and 5β-pregnane (17β-ethyletiocholane). It has a gonane core.
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of Nomenclature of Inorganic Chemistry. It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.
In organic chemistry, Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system, is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no more than ten ring members. Some common heterocyclic compounds have retained names that do not follow the Hantzsch–Widman pattern.
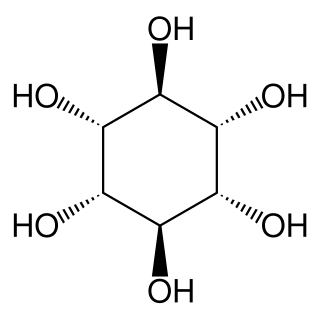
muco-Inositol is a critically important chemical in the gustatory (taste) modality of the mammalian nervous system. The generic form is coupled to a phospholipid of the outer lemma of the sensory neurons associated with the sodium ion sensitive channel of gustation.
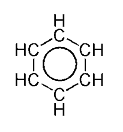
In chemistry, a parent structure is the structure of an unadorned ion or molecule from which derivatives can be visualized. Parent structures underpin systematic nomenclature and facilitate classification. Fundamental parent structures have one or no functional groups and often have various types of symmetry. Benzene is a chemical itself consisting of a hexagonal ring of carbon atoms with a hydrogen atom attached to each, and is the parent of many derivatives that have substituent atoms or groups replacing one or more of the hydrogens. Some parents are rare or nonexistent themselves, as in the case of porphine, though many simple and complex derivatives are known.
The International Union of Pure and Applied Chemistry publishes many books which contain its complete list of definitions. The definitions are divided into seven "colour books": Gold, Green, Blue, Purple, Orange, White, and Red. There is also an eighth book—the "Silver Book".
IUPAC Polymer Nomenclature are standardized naming conventions for polymers set by the International Union of Pure and Applied Chemistry (IUPAC) and described in their publication "Compendium of Polymer Terminology and Nomenclature", which is also known as the "Purple Book". Both the IUPAC and Chemical Abstracts Service (CAS) make similar naming recommendations for the naming of polymers.
A descriptor is in chemical nomenclature a prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.