
A thiol or thiol derivative is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol", where the first word deriving from Greek θεῖον (theion) meaning "sulfur".

Zinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This white salt is hygroscopic and even deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Thionyl chloride is an inorganic compound with the chemical formula SOCl
2. It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type: 2 A → A' + A", regardless of whether it is a redox or some other type of process.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols except the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
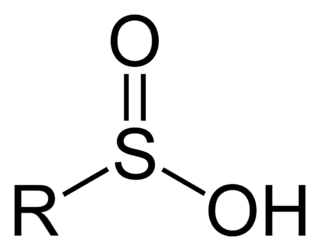
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
Sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.

A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.
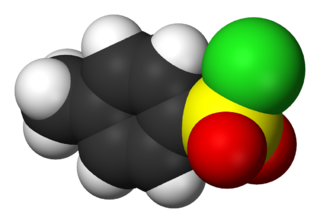
4-Toluenesulfonyl chloride (p-toluenesulfonyl chloride, toluene-p-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group.

Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6H6O3S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.
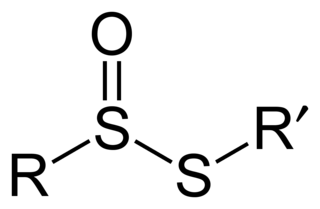
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R (R are organic substituents). Thiolsulfinates are also named as alkanethiosulfinic (or arenethiosulfinic) acid esters. They are the first member of a family of compounds containing an oxidized disulfide bond. Other members of this family include thiosulfonates (R-SO2-S-R), α-disulfoxides (R-S(O)-S(O)-R), sulfinyl sulfones (R-S(O)-SO2-R), and α-disulfones (R-SO2-SO2-R), all of which are known. The thiosulfinate group can occur in cyclic as well as acyclic structures.
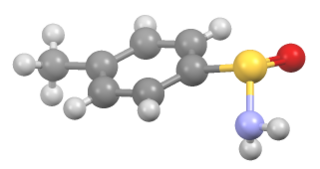
Sulfinamide is a functional group in organosulfur chemistry with the structural formula RS(O)NR'2. This functionality is composed of a sulfur-carbon (S-C) and sulfur-nitrogen (S-N) single bonds, as well as a sulfur-oxygen double bond (S=O), resulting in a tetravalent sulfur centre. As a non-bonding electron pair is also present on the sulfur, these compounds are also chiral. They are sometimes referred to as S-chiral sulfinamides. Sulfinamides are amides of sulfinic acid.
Thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form (RC OH) and a thiol form (RC SH). These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

In chemistry, the sulfonamide functional group (also spelled sulphonamide) is -S(=O)2-NH2, a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. The amine center is no longer basic. The S-N bond is cleaved only with difficulty. Because of the rigidity of the functional group, sulfonamides are typically crystalline. For this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.

















