
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles.

In chemistry, aromaticity is a property of cyclic (ring-shaped), typically planar (flat) molecular structures with pi bonds in resonance that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
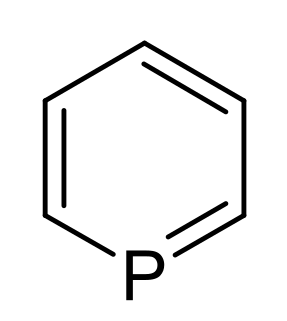
Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.

Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
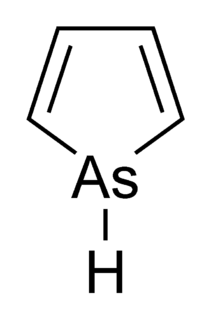
Arsole, also called arsenole or arsacyclopentadiene, is an organoarsenic compound with the formula C4H4AsH. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole. Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Lawesson's reagent (LR) is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.

Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula C4H4N2. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine rings, pyrimidine and pyrazine.

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of [C7H7]+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (see below). Its bromide and chloride salts can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively.

Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

Trithiapentalene is an organic bicyclic molecule containing two sulfur heterocycles. Its 10-π aromatic structure is similar to naphthalene. There has been a literature dispute about whether the connectivity among the three sulfur atoms is a case of rapid tautomerization between two valence tautomers or a 3-center 4-electron bond.
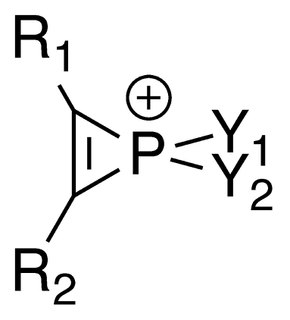
Phosphirenium ions are a series of organophosphorus compounds containing unsaturated three-membered ring phosphorus (V) heterocycles and σ*-aromaticity is believed to be present in such molecules. Many of the salts containing phosphirenium ions have been isolated and characterized by NMR spectroscopy and X-ray crystallography.
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.
















