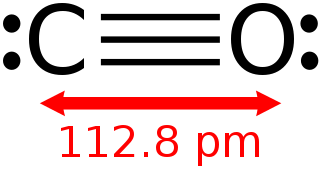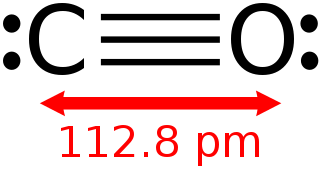
Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each cycle of such interactions." In many cases, the catalyst is a solid that upon irradiation with UV- or visible light generates electron–hole pairs that generate free radicals. Photocatalysts belong to three main groups; heterogeneous, homogeneous, and plasmonic antenna-reactor catalysts. The use of each catalysts depends on the preferred application and required catalysis reaction.
Polyketones are a family of high-performance thermoplastic polymers. The polar ketone groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for copolymer, 220 °C for terpolymer. Trade names include Poketone, Carilon, Karilon, Akrotek, and Schulaketon. Such materials also tend to resist solvents and have good mechanical properties. Unlike many other engineering plastics, aliphatic polyketones such as Shell Chemicals' Carilon are relatively easy to synthesize and can be derived from inexpensive monomers. Carilon is made with a palladium catalyst from ethylene and carbon monoxide. A small fraction of the ethylene is generally replaced with propylene to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S. in 1996, but discontinued it in 2000. Hyosung announced that they would launch production in 2015.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Photosensitizers are light absorbers that alter the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
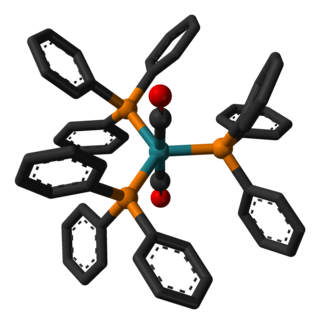
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center.

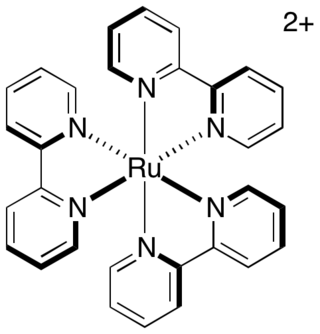
Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]Cl2. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
Photocatalytic water splitting is a process that uses photocatalysis for the dissociation of water (H2O) into hydrogen (H
2) and oxygen (O
2). Only light energy (photons), water, and a catalyst(s) are needed, since this is what naturally occurs in natural photosynthetic oxygen production and CO2 fixation. Photocatalytic water splitting is done by dispersing photocatalyst particles in water or depositing them on a substrate, unlike Photoelectrochemical cell, which are assembled into a cell with a photoelectrode.
The electrochemical reduction of carbon dioxide, also known as CO2RR, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It represents one potential step in the broad scheme of carbon capture and utilization.

Andrew Bruce Bocarsly is currently a professor at Princeton University, New Jersey. His primary research interests lie in physical inorganic chemistry. He conducts research in electrochemistry, photochemistry, solids state chemistry, and fuel cells, and is known for his work on alternate energy solutions involving processes and materials for photo-reduction and electro-reduction.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.
Photoelectrochemical reduction of carbon dioxide, also known as photoelectrolysis of carbon dioxide, is a chemical process whereby carbon dioxide is reduced to carbon monoxide or hydrocarbons by the energy of incident light. This process requires catalysts, most of which are semiconducting materials. The feasibility of this chemical reaction was first theorised by Giacomo Luigi Ciamician, an Italian photochemist. Already in 1912 he stated that "[b]y using suitable catalyzers, it should be possible to transform the mixture of water and carbon dioxide into oxygen and methane, or to cause other endo-energetic processes."

Graphitic carbon nitride (g-C3N4) is a family of carbon nitride compounds with a general formula near to C3N4 (albeit typically with non-zero amounts of hydrogen) and two major substructures based on heptazine and poly(triazine imide) units which, depending on reaction conditions, exhibit different degrees of condensation, properties and reactivities.
A solar fuel is a synthetic chemical fuel produced from solar energy. Solar fuels can be produced through photochemical, photobiological, and electrochemical reactions.
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.

Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.