
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.
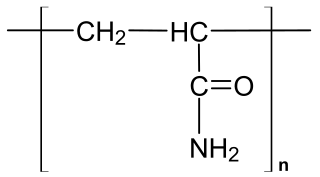
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries.
![<span class="mw-page-title-main">Polyacetylene</span> Organic polymer made of the repeating unit [C2H2]](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png/320px-Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png)
Polyacetylene usually refers to an organic polymer with the repeating unit [C2H2]n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics. This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
Aluminium chlorohydrate is a group of water-soluble, specific aluminium salts having the general formula AlnCl3n−m(OH)m. It is used in cosmetics as an antiperspirant and as a coagulant in water purification.

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.

Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.
Electrocoagulation (EC) is a technique used for wastewater treatment, wash water treatment, industrially processed water, and medical treatment. Electrocoagulation has become a rapidly growing area of wastewater treatment due to its ability to remove contaminants that are generally more difficult to remove by filtration or chemical treatment systems, such as emulsified oil, total petroleum hydrocarbons, refractory organics, suspended solids, and heavy metals. There are many brands of electrocoagulation devices available, and they can range in complexity from a simple anode and cathode to much more complex devices with control over electrode potentials, passivation, anode consumption, cell REDOX potentials as well as the introduction of ultrasonic sound, ultraviolet light and a range of gases and reactants to achieve so-called Advanced Oxidation Processes for refractory or recalcitrant organic substances.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.

Nanoporous materials consist of a regular organic or inorganic bulk phase in which a porous structure is present. Nanoporous materials exhibit pore diameters that are most appropriately quantified using units of nanometers. The diameter of pores in nanoporous materials is thus typically 100 nanometers or smaller. Pores may be open or closed, and pore connectivity and void fraction vary considerably, as with other porous materials. Open pores are pores that connect to the surface of the material whereas closed pores are pockets of void space within a bulk material. Open pores are useful for molecular separation techniques, adsorption, and catalysis studies. Closed pores are mainly used in thermal insulators and for structural applications.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).
Clarifying agents are used to remove suspended solids from liquids by inducing flocculation, causing the solids to form larger aggregates that can be easily removed after they either float to the surface or sink to the bottom of the containment vessel.

SNF is one of the largest manufacturers of polyacrylamides. These water-soluble polymers are used as flocculants and coagulants in solid/water separation to recycle water, rheology modifiers and friction reducers. These functionalities have many uses where water is used, in drinking water production, wastewater treatment, mining, paper, enhanced oil recovery, hydraulic fracturing, agriculture, textile and cosmetics.

In water treatment, coagulation and flocculation involve the addition of compounds that promote the clumping of fine floc into larger floc so that they can be more easily separated from the water. Coagulation is a chemical process that involves neutralization of charge whereas flocculation is a physical process and does not involve neutralization of charge. The coagulation-flocculation process can be used as a preliminary or intermediary step between other water or wastewater treatment processes like filtration and sedimentation. Iron and aluminium salts are the most widely used coagulants but salts of other metals such as titanium and zirconium have been found to be highly effective as well.
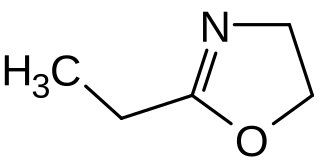
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
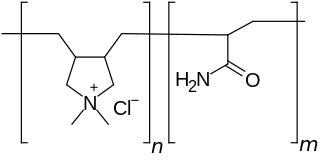
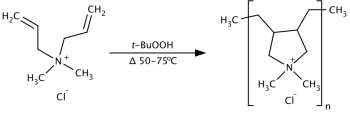
Polyquaternium-7 is an organic compound in the polyquaternium class of chemicals and used in the personal care industry. It is the copolymer of acrylamide and the quaternary ammonium salt diallyldimethylammonium chloride.





![<span class="mw-page-title-main">Polyacetylene</span> Organic polymer made of the repeating unit [C2H2]](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png/320px-Cis-and-trans-polyacetylene-chains-symmetric-8-based-on-xtals-3D-bs-17.png)







