![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)
In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane. They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, grease, rubber, resin, and caulk.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.
A cermet is a composite material composed of ceramic and metal materials.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers in solution into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.

Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat, or at lower temperatures using precipitation reactions from high-purity chemical solutions. The term includes the purification of raw materials, the study and production of the chemical compounds concerned, their formation into components and the study of their structure, composition and properties.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.

In materials science ceramic matrix composites (CMCs) are a subgroup of composite materials and a subgroup of ceramics. They consist of ceramic fibers embedded in a ceramic matrix. The fibers and the matrix both can consist of any ceramic material, including carbon and carbon fibers.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).

Silicon oxynitride is a ceramic material with the chemical formula SiOxNy. While in amorphous forms its composition can continuously vary between SiO2 (silica) and Si3N4 (silicon nitride), the only known intermediate crystalline phase is Si2N2O. It is found in nature as the rare mineral sinoite in some meteorites and can be synthesized in the laboratory.

Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used as precursors to silicon carbide. The simplest polysilane would be (SiH2)n, which is mainly of theoretical, not practical interest.
SiC–SiC matrix composite is a particular type of ceramic matrix composite (CMC) which have been accumulating interest mainly as high temperature materials for use in applications such as gas turbines, as an alternative to metallic alloys. CMCs are generally a system of materials that are made up of ceramic fibers or particles that lie in a ceramic matrix phase. In this case, a SiC/SiC composite is made by having a SiC matrix phase and a fiber phase incorporated together by different processing methods. Outstanding properties of SiC/SiC composites include high thermal, mechanical, and chemical stability while also providing high strength to weight ratio.
Robocasting is an additive manufacturing technique analogous to Direct Ink Writing and other extrusion-based 3D-printing techniques in which a filament of a paste-like material is extruded from a small nozzle while the nozzle is moved across a platform. The object is thus built by printing the required shape layer by layer. The technique was first developed in the United States in 1996 as a method to allow geometrically complex ceramic green bodies to be produced by additive manufacturing. In robocasting, a 3D CAD model is divided up into layers in a similar manner to other additive manufacturing techniques. The material is then extruded through a small nozzle as the nozzle's position is controlled, drawing out the shape of each layer of the CAD model. The material exits the nozzle in a liquid-like state but retains its shape immediately, exploiting the rheological property of shear thinning. It is distinct from fused deposition modelling as it does not rely on the solidification or drying to retain its shape after extrusion.
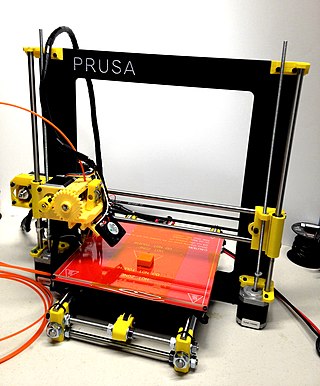
Fused filament fabrication (FFF), also known as fused deposition modeling, or filament freeform fabrication, is a 3D printing process that uses a continuous filament of a thermoplastic material. Filament is fed from a large spool through a moving, heated printer extruder head, and is deposited on the growing work. The print head is moved under computer control to define the printed shape. Usually the head moves in two dimensions to deposit one horizontal plane, or layer, at a time; the work or the print head is then moved vertically by a small amount to begin a new layer. The speed of the extruder head may also be controlled to stop and start deposition and form an interrupted plane without stringing or dribbling between sections. "Fused filament fabrication" was coined by the members of the RepRap project to give an acronym (FFF) that would be legally unconstrained in its use.
Oxycarbide glass, also referred to as silicon oxycarbide, is a type of glass that contains oxygen and carbon in addition to silicon dioxide. It is created by substituting some oxygen atoms with carbon atoms. This glass may contain particles of amorphous carbon, and silicon carbide. SiOC materials of varying stoichiometery are attractive owing to their generally high density, hardness and high service temperatures. Through diverse forming techniques high performance parts in complex shapes can be achieved. Unlike pure SiC, the versatile stoichiometry of SiOC offers further avenues to tune physical properties through appropriate selection of processing parameters.
Gurpreet Singh is a professor of Mechanical and Nuclear Engineering at [Kansas State University]. He is endowed by the Harold O. and Jane C. Massey Neff Professorship in Mechanical Engineering. Singh was born in Ludhiana, India; he currently resides in the United States.
The term preceramic polymer refers to one of various polymeric compounds, which through pyrolysis under appropriate conditions are converted to ceramic compounds, having high thermal and chemical stability. Ceramics resulting from the pyrolysis of preceramic polymers are known as polymer derived ceramics, or PDCs. Polymer derived ceramics are most often silicon based and include silicon carbide, silicon oxycarbide, silicon nitride and silicon oxynitride. Such PDCs are most commonly amorphous, lacking long-range crystalline order.
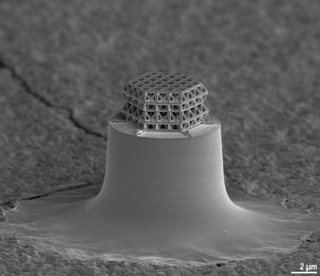
A nanolattice is a synthetic porous material consisting of nanometer-size members patterned into an ordered lattice structure, like a space frame. The nanolattice is a newly emerged material class that has been rapidly developed over the last decade. Nanolattices redefine the limits of the material property space. Despite being composed of 50-99% of air, nanolattices are very mechanically robust because they take advantage of size-dependent properties that we generally see in nanoparticles, nanowires, and thin films. The most typical mechanical properties of nanolattices include ultrahigh strength, damage tolerance, and high stiffness. Thus, nanolattices have a wide range of applications.

Katherine T. Faber is an American materials scientist and one of the world's foremost experts in ceramic engineering, material strengthening, and ultra-high temperature materials. Faber is the Simon Ramo Professor of Materials Science at the California Institute of Technology (Caltech). She was previously the Walter P. Murphy Professor and department chair of Materials Science and Engineering at the McCormick School of Engineering and Applied Science at Northwestern University.



![<span class="mw-page-title-main">Silicone</span> Family of polymers of the repeating form [R2Si–O–SiR2]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Caulking.jpg/320px-Caulking.jpg)










