Related Research Articles

A polymer (;) is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.
Rheology is the study of the flow of matter, primarily in a fluid state, but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applied force. Rheology is a branch of physics, and it is the science that deals with the deformation and flow of materials, both solids and liquids.

A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady state, although the liquid phase may still diffuse through this system. A gel has been defined phenomenologically as a soft, solid or solid-like material consisting of two or more components, one of which is a liquid, present in substantial quantity.

The guar or cluster bean, with the botanical name Cyamopsis tetragonoloba, is an annual legume and the source of guar gum. It is also known as gavar, gawar, or guvar bean.

Guar gum, also called guaran, is a galactomannan polysaccharide extracted from guar beans that has thickening and stabilizing properties useful in food, feed, and industrial applications. The guar seeds are mechanically dehusked, hydrated, milled and screened according to application. It is typically produced as a free-flowing, off-white powder.
Gel permeation chromatography (GPC) is a type of size-exclusion chromatography (SEC), that separates high molecular weight or colloidal analytes on the basis of size or diameter, typically in organic solvents. The technique is often used for the analysis of polymers. As a technique, SEC was first developed in 1955 by Lathe and Ruthven. The term gel permeation chromatography can be traced back to J.C. Moore of the Dow Chemical Company who investigated the technique in 1964. The proprietary column technology was licensed to Waters Corporation, who subsequently commercialized this technology in 1964. GPC systems and consumables are now also available from a number of manufacturers. It is often necessary to separate polymers, both to analyze them as well as to purify the desired product.
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.
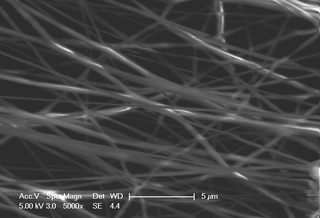
Electrospinning is a fiber production method that uses electric force to draw charged threads of polymer solutions or polymer melts up to fiber diameters in the order of some hundred nanometers. Electrospinning shares characteristics of both electrospraying and conventional solution dry spinning of fibers. The process does not require the use of coagulation chemistry or high temperatures to produce solid threads from solution. This makes the process particularly suited to the production of fibers using large and complex molecules. Electrospinning from molten precursors is also practiced; this method ensures that no solvent can be carried over into the final product.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol-gel process is used to produce ceramic nanoparticles.

In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally-occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.
An ionomer is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups.
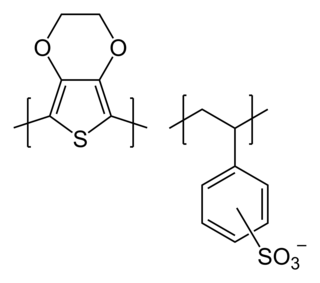
poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) is a polymer mixture of two ionomers. One component in this mixture is made up of polystyrene sulfonate which is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) (PEDOT) is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

Flory–Huggins solution theory is a lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixing. The result is an equation for the Gibbs free energy change for mixing a polymer with a solvent. Although it makes simplifying assumptions, it generates useful results for interpreting experiments.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.
The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible in all proportions. The word lower indicates that the LCST is a lower bound to a temperature interval of partial miscibility, or miscibility for certain compositions only.
Spinning is a manufacturing process for creating polymer fibers. It is a specialized form of extrusion that uses a spinneret to form multiple continuous filaments.

A proppant is a solid material, typically sand, treated sand or man-made ceramic materials, designed to keep an induced hydraulic fracture open, during or following a fracturing treatment, most commonly for unconventional reservoirs. It is added to a fracking fluid which may vary in composition depending on the type of fracturing used, and can be gel, foam or slickwater–based. In addition, there may be unconventional fracking fluids. Fluids make tradeoffs in such material properties as viscosity, where more viscous fluids can carry more concentrated proppant; the energy or pressure demands to maintain a certain flux pump rate that will conduct the proppant appropriately; pH, various rheological factors, among others. In addition, fluids may be used in low-volume well stimulation of high-permeability sandstone wells to the high-volume operations such as shale gas and tight gas that use millions of gallons of water per well.
Mechanics of gelation describes processes relevant to sol-gel process.
References
- ↑ Teraoka, Iwao (2002). Polymer solutions: an introduction to physical properties (PDF). John Wiley & Sons. ISBN 978-0-471-38929-3.
- ↑ Chemical Fabrics and Film Association [CFFA] (n.d.). "Plasticizer migration" (PDF). CFFA Performance Products Division. Archived (PDF) from the original on February 14, 2017. Retrieved August 11, 2017.
- ↑ "Polymer solutions".
- ↑ Danner, Ronald P.; High, Martin S. (1993). Handbook of polymer solution thermodynamics. New York: Design Institute for Physical Property Data (DIPPR), American Institute of Chemical Engineers (AIChE). ISBN 0-8169-0579-7.
- ↑ Schubert, Dirk W. (2020). "Novel Theoretical Self‐Consistent Mean‐Field Approach to Describe the Conductivity of Carbon Fiber‐Filled Thermoplastics: Part III—Application of the Concept to Mechanical Properties of Composites and Polymer Solutions". Advanced Engineering Materials. 22 (9): 2000171. doi: 10.1002/adem.202000171 . ISSN 1438-1656.
- ↑ Chang, Shun-Chi; Yang, Yang (1999). "Polymer solution light-emitting devices". Applied Physics Letters. 74 (2081): 2081–2083. doi:10.1063/1.123764.
- ↑ USpatent 5488083 A,Kinsey, III, E. Wayne; Sharif, Sharif& Harry, David N.,"Method of gelling a guar or derivatized guar polymer solution utilized to perform a hydraulic fracturing operation",issued 1996-01-30, assigned to Benchmark Research and Technology, Inc.