
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface.

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively.

Methylene diphenyl diisocyanate (MDI) is an aromatic diisocyanate. Three isomers are common, varying by the positions of the isocyanate groups around the rings: 2,2′-MDI, 2,4′-MDI, and 4,4′-MDI. The 4,4′ isomer is most widely used, and is also known as 4,4′-diphenylmethane diisocyanate. This isomer is also known as Pure MDI. MDI reacts with polyols in the manufacture of polyurethane. It is the most produced diisocyanate, accounting for 61.3% of the global market in the year 2000.

In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally-occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.
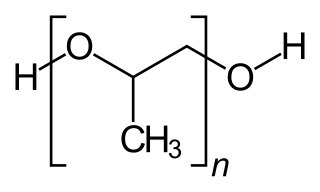
Polypropylene glycol or polypropylene oxide is the polymer of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG) H S Code 3907.2000. The term polypropylene glycol or PPG is reserved for polymer of low- to medium-range molar mass when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" is used for high-molar-mass polymer when end-groups no longer affect polymer properties. Between 60 and 70% of propylene oxide is converted to polyether polyols by the process called alkoxylation.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers that consist of materials with both thermoplastic and elastomeric properties. While most elastomers are thermosets, thermoplastics are in contrast relatively easy to use in manufacturing, for example, by injection moulding. Thermoplastic elastomers show advantages typical of both rubbery materials and plastic materials. The benefit of using thermoplastic elastomers is the ability to stretch to moderate elongations and return to its near original shape creating a longer life and better physical range than other materials. The principal difference between thermoset elastomers and thermoplastic elastomers is the type of cross-linking bond in their structures. In fact, crosslinking is a critical structural factor which imparts high elastic properties.
Polyether block amide or PEBA is a thermoplastic elastomer (TPE). It is known under the tradename of PEBAX® (Arkema) and VESTAMID® E. It is a block copolymer obtained by polycondensation of a carboxylic acid polyamide with an alcohol termination polyether, PEG). The general chemical structure is:
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.
Thermoplastic polyurethane (TPU) is any of a class of polyurethane plastics with many properties, including elasticity, transparency, and resistance to oil, grease, and abrasion. Technically, they are thermoplastic elastomers consisting of linear segmented block copolymers composed of hard and soft segments.
Polyoxetane (POX), or poly(oxetane), is synthetic organic heteroatomic thermoplastic polymer with molecular formula (–OCH2CH2CH2–)n. It is polymerized from oxetane monomer, which is a four-membered cyclic ether.

![Terathane container owned by DuPont (before the business was sold to Invista). Container [( 22T6 )] CCRU 164132(1) [( Pictures taken in Japan )] .jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/3/33/Container_%E3%80%90_22T6_%E3%80%91_CCRU_164132%281%29_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg/220px-Container_%E3%80%90_22T6_%E3%80%91_CCRU_164132%281%29_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg)












