A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics. However, material surfaces can also have bactericidal properties based solely on their physical surface structure, as for example biomaterials like insect wings.
Ethanol is a chemical compound, a simple alcohol with the chemical formula C
2H
6O. Its formula can be also written as CH
3−CH
2−OH or C
2H
5OH, and is often abbreviated as EtOH. Ethanol is a volatile, flammable, colorless liquid with a slight characteristic odor. It is a psychoactive substance and is the principal active ingredient found in alcoholic drinks.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is a key intermediate in several metabolic pathways throughout the cell.

Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Precipitation defined as the creation of a solid from a solution. When the reaction occurs in a liquid solution, the solid formed is called the 'precipitate'. The chemical that causes the solid to form is called the 'precipitant'. Without sufficient force of gravity (settling) to bring the solid particles together, the precipitate remains in suspension. After sedimentation, especially when using a centrifuge to press it into a compact mass, the precipitate may be referred to as a 'pellet'. Precipitation can be used as a medium. The precipitate-free liquid remaining above the solid is called the 'supernate' or 'supernatant'. Powders derived from precipitation have also historically been known as 'flowers'. When the solid appears in the form of cellulose fibers which have been through chemical processing, the process is often referred to as regeneration.

Phthalic anhydride is the organic compound with the formula :-C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant toward hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.

Potassium dichromate, K
2Cr
2O
7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in the laboratory because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
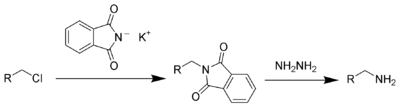
The Gabriel synthesis is a chemical reaction that transforms primary alkyl halides into primary amines. Traditionally, the reaction uses potassium phthalimide. The reaction is named after the German chemist Siegmund Gabriel.

Potassium acetate (CH3COOK) is the potassium salt of acetic acid.
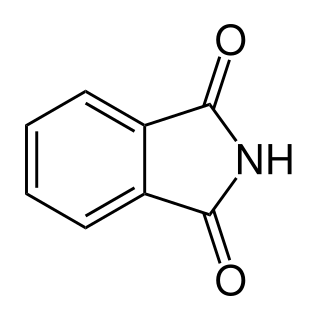
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor to other organic compounds as a masked source of ammonia.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The ReH2−
9 anion is a rare example of a coordination complex bearing only hydride ligands.
The haloform reaction is a chemical reaction where a haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of a methyl ketone (RCOCH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform (CHCl3), bromoform (CHBr3), or iodoform (CHI3) and also cyanide.

Hydroxy-alpha sanshool is a molecule found in plants from the genus Zanthoxylum. It is believed to be responsible for the numbing and tingling sensation caused by eating food cooked with Sichuan peppercorns.

Sodium ferrioxalate is a chemical compound with the formula Na
3Fe(C
2O
4)
3. It is also called sodium oxalatoferrate or sodium trisoxalatoferrate.
Georges-Simon Serullas was a professor of pharmacy notable for being the first to publish a work on Iodoform, an early antiseptic and disinfectant.