Related Research Articles
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates cell from the external environment or creates intracellular compartments. Biological membranes, in the form of eukaryotic cell membranes, consist of a phospholipid bilayer with embedded, integral and peripheral proteins used in communication and transportation of chemicals and ions. The bulk of lipid in a cell membrane provides a fluid matrix for proteins to rotate and laterally diffuse for physiological functioning. Proteins are adapted to high membrane fluidity environment of lipid bilayer with the presence of an annular lipid shell, consisting of lipid molecules bound tightly to surface of integral membrane proteins. The cell membranes are different from the isolating tissues formed by layers of cells, such as mucous membranes, basement membranes, and serous membranes.

Facilitated diffusion is the process of spontaneous passive transport of molecules or ions across a biological membrane via specific transmembrane integral proteins. Being passive, facilitated transport does not directly require chemical energy from ATP hydrolysis in the transport step itself; rather, molecules and ions move down their concentration gradient reflecting its diffusive nature.

Oxidative phosphorylation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing the chemical energy stored within in order to produce adenosine triphosphate (ATP). In most eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because the energy of the double bond of oxygen is so much higher than the energy of the double bond in carbon dioxide or in pairs of single bonds in organic molecules observed in alternative fermentation processes such as anaerobic glycolysis.
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:

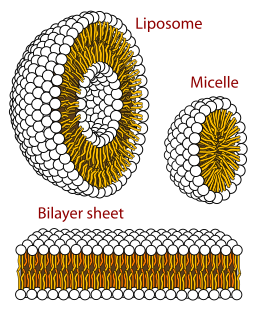
The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

Peripheral membrane proteins are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.

Nonactin is a member of a family of naturally occurring cyclic ionophores known as the macrotetrolide antibiotics. The other members of this homologous family are monactin, dinactin, trinactin and tetranactin which are all neutral ionophoric substances and higher homologs of nonactin. Collectively, this class is known as the nactins. Nonactin is soluble in methanol, dichloromethane, ethyl acetate and DMSO, but insoluble in water.
In cellular biology, membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes, which are lipid bilayers that contain proteins embedded in them. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they can be permeable to certain substances but not to others.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An example of this would be the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found.

Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes.

An amphiphile is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. This forms the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of a prolate molecule are called bolaamphiphilic. Common amphiphilic substances are soaps, detergents, and lipoproteins.
Flippases are transmembrane lipid transporter proteins located in the membrane which belong to ABC transporter or P4-type ATPase families. They are responsible for aiding the movement of phospholipid molecules between the two leaflets that compose a cell's membrane. The possibility of active maintenance of an asymmetric distribution of molecules in the phospholipid bilayer was predicted in the early 1970s by Mark Bretscher. Although phospholipids diffuse rapidly in the plane of the membrane, their polar head groups cannot pass easily through the hydrophobic center of the bilayer, limiting their diffusion in this dimension. Some flippases - often instead called scramblases - are energy-independent and bidirectional, causing reversible equilibration of phospholipid between the two sides of the membrane, whereas others are energy-dependent and unidirectional, using energy from ATP hydrolysis to pump the phospholipid in a preferred direction. Flippases are described as transporters that move lipids from the exoplasmic to the cytosolic face, while floppases transport in the reverse direction.
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion of proteins and other bio-molecules within the membrane, there-by affecting the functions of these things.

Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism. CCCP affects the protein synthesis reactions in seedling mitochondria. CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally.
Transmembrane channels, also called membrane channels, are pores within a lipid bilayer. The channels can be formed by protein complexes that run across the membrane or by peptides. They may cross the cell membrane, connecting the cytosol, or cytoplasm, to the extracellular matrix. Transmembrane channels are also found in the membranes of organelles including the nucleus, the endoplasmic reticulum, the Golgi apparatus, mitochondria, chloroplasts, and lysosomes.

The presence of ethanol can lead to the formations of non-lamellar phases also known as non-bilayer phases. Ethanol has been recognized as being an excellent solvent in an aqueous solution for inducing non-lamellar phases in phospholipids. The formation of non-lamellar phases in phospholipids is not completely understood, but it is significant that this amphiphilic molecule is capable of doing so. The formation of non-lamellar phases is significant in biomedical studies which include drug delivery, the transport of polar and non-polar ions using solvents capable of penetrating the biomembrane, increasing the elasticity of the biomembrane when it is being disrupted by unwanted substances and functioning as a channel or transporter of biomaterial.
Hydrophobic mismatch is the difference between the thicknesses of hydrophobic regions of a transmembrane protein and of the biological membrane it spans. In order to avoid unfavorable exposure of hydrophobic surfaces to water, the hydrophobic regions of transmembrane proteins are expected to have approximately the same thickness as the hydrophobic region of the surrounding lipid bilayer. Nevertheless, the same membrane protein can be encountered in bilayers of different thickness. In eukaryotic cells, the plasma membrane is thicker than the membranes of the endoplasmic reticulum. Yet all proteins that are abundant in the plasma membrane are initially integrated into the endoplasmic reticulum upon synthesis on ribosomes. Transmembrane peptides or proteins and surrounding lipids can adapt to the hydrophobic mismatch by different means.

The cell membrane is the semipermeable membrane of a cell that surrounds and encloses its contents of cytoplasm and nucleoplasm. The cell membrane separates the cell from the surrounding interstitial fluid, the main component of the extracellular fluid.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton motive force, but the proton motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.
References
- ↑ http://biom.3322.org:2966/ebook1/biophy/Fundamental%20Principles%20of%20Membrane%20Biophysics.pdf%5B%5D (accessed 19th Nov 2008)
- ↑ Nicholls, David G. & Ferguson, Stuart J. (2002). Bioenergetics 3. London: Academic Press. ISBN 978-0-12-518121-1.
- ↑ Chopineaux-Courtois V, Reymond F, Bouchard G, Carrupt PA, Testa B, Girault HH (February 1999). "Effects of Charge and Intramolecular Structure on the Lipophilicity of Nitrophenols". J. Am. Chem. Soc. 121 (8): 1743–1747. doi:10.1021/ja9836139.
- ↑ Tharmalingam N, Jayamani E, Rajamuthiah R, Castillo D, Fuchs BB, Kelso MJ, Mylonakis E (2017). "Activity of a novel protonophore against methicillin-resistant Staphylococcus aureus". Future Med. Chem. 9 (12): 1401–1411. doi:10.4155/fmc-2017-0047. PMC 5941710 . PMID 28771026.
- ↑ Ozaki S, Kano K, Shirai O (August 2008). "Electrochemical elucidation on the mechanism of uncoupling caused by hydrophobic weak acids". Phys Chem Chem Phys. 10 (30): 4449–55. Bibcode:2008PCCP...10.4449O. doi:10.1039/b803458c. PMID 18654685.