
An alum is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula XAl(SO
4)
2·12 H
2O, such that X is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula KAl(SO
4)
2·12 H
2O. Other alums are named after the monovalent ion, such as sodium alum and ammonium alum.

Carl Wilhelm Scheele was a Swedish German pharmaceutical chemist.

Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.

Cryolite (Na3AlF6, sodium hexafluoroaluminate) is an uncommon mineral identified with the once-large deposit at Ivittuut on the west coast of Greenland, mined commercially until 1987.

Carl Friedrich Christian Mohs was a German chemist and mineralogist. He was the creator of the Mohs scale of mineral hardness. Mohs also introduced a classification of the crystal forms in crystal systems independently of Christian Samuel Weiss.

Vanadinite is a mineral belonging to the apatite group of phosphates, with the chemical formula Pb5(VO4)3Cl. It is one of the main industrial ores of the metal vanadium and a minor source of lead. A dense, brittle mineral, it is usually found in the form of red hexagonal crystals. It is an uncommon mineral, formed by the oxidation of lead ore deposits such as galena. First discovered in 1801 in Mexico, vanadinite deposits have since been unearthed in South America, Europe, Africa, and North America.

Martin Heinrich Klaproth was a German chemist. He trained and worked for much of his life as an apothecary, moving in later life to the university. His shop became the second-largest apothecary in Berlin, and the most productive artisanal chemical research center in Europe.

Anglesite is a lead sulfate mineral with the chemical formula PbSO4. It occurs as an oxidation product of primary lead sulfide ore, galena. Anglesite occurs as prismatic orthorhombic crystals and earthy masses, and is isomorphous with barite and celestine. It contains 74% of lead by mass and therefore has a high specific gravity of 6.3. Anglesite's color is white or gray with pale yellow streaks. It may be dark gray if impure.

Mimetite is a lead arsenate chloride mineral (Pb5(AsO4)3Cl) which forms as a secondary mineral in lead deposits, usually by the oxidation of galena and arsenopyrite. The name derives from the Greek Μιμητής mimetes, meaning "imitator" and refers to mimetite's resemblance to the mineral pyromorphite. This resemblance is not coincidental, as mimetite forms a mineral series with pyromorphite (Pb5(PO4)3Cl) and with vanadinite (Pb5(VO4)3Cl). Notable occurrences are Mapimi, Durango, Mexico and Tsumeb, Namibia.

Johann Friedrich Ludwig Hausmann was a German mineralogist.

Descloizite is a rare mineral species consisting of basic lead and zinc vanadate, (Pb, Zn)2(OH)VO4, crystallizing in the orthorhombic crystal system and isomorphous with olivenite. Appreciable gallium and germanium may also be incorporated into the crystal structure.

Stolzite is a mineral, a lead tungstate; with the formula PbWO4. It is similar to, and often associated with, wulfenite which is the same chemical formula except that the tungsten is replaced by molybdenum. Stolzite crystallizes in the tetragonal crystal system and is dimorphous with the monoclinic form raspite.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

Tsumebite is a rare phosphate mineral named in 1912 after the locality where it was first found, the Tsumeb mine in Namibia, well known to mineral collectors for the wide range of minerals found there. Tsumebite is a compound phosphate and sulfate of lead and copper, with hydroxyl, formula Pb2Cu(PO4)(SO4)(OH). There is a similar mineral called arsentsumebite, where the phosphate group PO4 is replaced by the arsenate group AsO4, giving the formula Pb2Cu(AsO4)(SO4)(OH). Both minerals are members of the brackebuschite group.

Mendipite is a rare mineral that was named in 1939 for the locality where it is found, the Mendip Hills in Somerset, England. It is an oxyhalide of lead with formula Pb3Cl2O2.
The Tabataud Quarry is situated in the northwestern French Massif Central. The quarry used to be mined for its granodiorite.
Boron phosphate is an inorganic compound with the chemical formula BPO4. The simplest way of producing it is the reaction of phosphoric acid and boric acid. It is a white infusible solid that evaporates above 1450 °C.

Mottramite is an orthorhombic anhydrous vanadate hydroxide mineral, PbCu(VO4)(OH), at the copper end of the descloizite subgroup. It was formerly called cuprodescloizite or psittacinite (this mineral characterized in 1868 by Frederick Augustus Genth). Duhamelite is a calcium- and bismuth-bearing variety of mottramite, typically with acicular habit.

Arsendescloizite is a lead-zinc mineral, approved by the IMA in 1982. It is an arsenate analog of descloizite. Its first description was published in 1982.
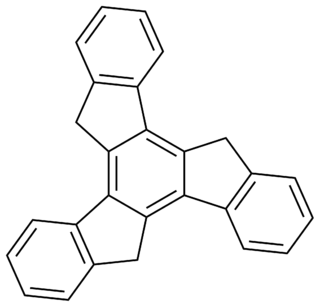
Truxene is a polycyclic aromatic hydrocarbon. The molecule can be thought of as being made up of three fluorene units arranged symmetrically and sharing a common central benzene. Truxene is solid, and it is slightly soluble in water.






















