
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process.
In chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes. In chemical reaction engineering, "yield", "conversion" and "selectivity" are terms used to describe ratios of how much of a reactant was consumed (conversion), how much desired product was formed (yield) in relation to the undesired product (selectivity), represented as X, Y, and S.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.

Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula (C6H5CO)2, generally abbreviated (PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.
Solar chemical refers to a number of possible processes that harness solar energy by absorbing sunlight in a chemical reaction. The idea is conceptually similar to photosynthesis in plants, which converts solar energy into the chemical bonds of glucose molecules, but without using living organisms, which is why it is also called artificial photosynthesis.
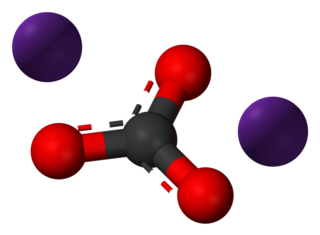
Caesium carbonate or cesium carbonate is a white crystalline solid compound. Caesium carbonate has a high solubility in polar solvents such as water, alcohol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium and sodium carbonates, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.

Atomic carbon, systematically named carbon and λ0-methane, is a colourless gaseous inorganic chemical with the chemical formula C. It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation.
In organic chemistry, the Ei mechanism, also known as a thermal syn elimination or a pericyclic syn elimination, is a special type of elimination reaction in which two vicinal (adjacent) substituents on an alkane framework leave simultaneously via a cyclic transition state to form an alkene in a syn elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents, unlike regular eliminations, which require an acid or base, or would in many cases involve charged intermediates. This reaction mechanism is often found in pyrolysis.
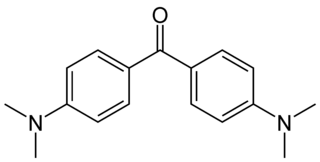
Michler's ketone is an organic compound with the formula of [(CH3)2NC6H4]2CO. This electron-rich derivative of benzophenone is an intermediate in the production of dyes and pigments, for example Methyl violet. It is also used as a photosensitizer. It is named after the German chemist Wilhelm Michler.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.

[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
The Buchner–Curtius–Schlotterbeck reaction is the reaction of aldehydes or ketones with aliphatic diazoalkanes to form homologated ketones. It was first described by Eduard Buchner and Theodor Curtius in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann in 1895 and Viktor Meyer in 1905. The reaction has since been extended to the synthesis of β-keto esters from the condensation between aldehydes and diazo esters. The general reaction scheme is as follows:
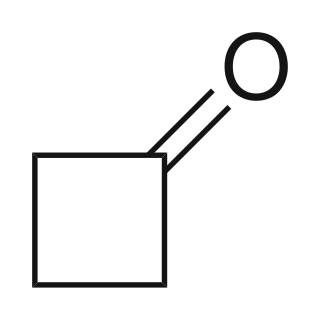
Cyclobutanone is an organic compound with molecular formula (CH2)3CO. It is a four-membered cyclic ketone (cycloalkanone). It is a colorless volatile liquid at room temperature. Since cyclopropanone is highly sensitive, cyclobutanone is the smallest easily handled cyclic ketone.

Lithium tetrahydridogallate is the inorganic compound with formula LiGaH4. It is a white solid similar to but less thermally robust than lithium aluminium hydride.
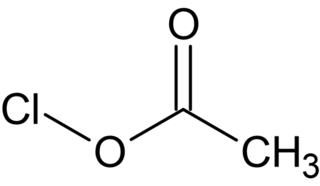
Acetyl hypochlorite, also known as chlorine acetate, is a chemical compound with the formula CH3COOCl. It is a photosensitive colorless liquid that is a short lived intermediate in the Hunsdiecker reaction.


















