Related Research Articles

A magnetometer is a device that measures magnetic field or magnetic dipole moment. Different types of magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location. A compass is one such device, one that measures the direction of an ambient magnetic field, in this case, the Earth's magnetic field. Other magnetometers measure the magnetic dipole moment of a magnetic material such as a ferromagnet, for example by recording the effect of this magnetic dipole on the induced current in a coil.
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.
Carbon-13 (C13) nuclear magnetic resonance is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the 13
C
isotope. The main carbon isotope, 12
C
is not detected. Although much less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds.
Nuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore, Angela Gronenborn at the NIH, and Gerhard Wagner at Harvard University, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequency (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).
Field Cycling (FC) is a measurement method which uses variable magnetic fields to measure the magnetization of a sample. Fast Field Cycling describes the same method but uses fast switchable magnetic fields.
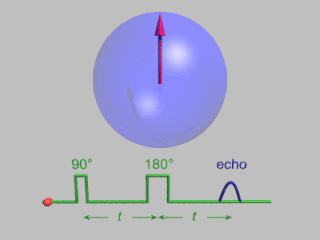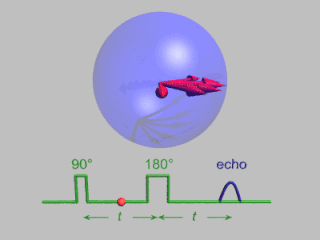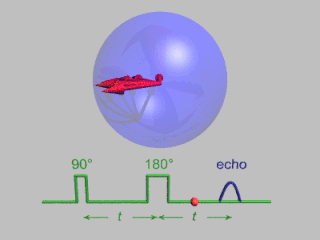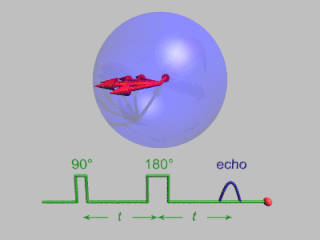
In magnetic resonance, a spin echo or Hahn echo is the refocusing of spin magnetisation by a pulse of resonant electromagnetic radiation. Modern nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) make use of this effect.

In physics, the spin–spin relaxation is the mechanism by which Mxy, the transverse component of the magnetization vector, exponentially decays towards its equilibrium value in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI). It is characterized by the spin–spin relaxation time, known as T2, a time constant characterizing the signal decay. It is named in contrast to T1, the spin–lattice relaxation time. It is the time it takes for the magnetic resonance signal to irreversibly decay to 37% (1/e) of its initial value after its generation by tipping the longitudinal magnetization towards the magnetic transverse plane. Hence the relation
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.

Zero- to ultralow-field (ZULF) NMR is the acquisition of nuclear magnetic resonance (NMR) spectra of chemicals with magnetically active nuclei in an environment carefully screened from magnetic fields. ZULF NMR experiments typically involve the use of passive or active shielding to attenuate Earth’s magnetic field. This is in contrast to the majority of NMR experiments which are performed in high magnetic fields provided by superconducting magnets. In ZULF experiments the dominant interactions are nuclear spin-spin couplings, and the coupling between spins and the external magnetic field is a perturbation to this. There are a number of advantages to operating in this regime: magnetic-susceptibility-induced line broadening is attenuated which reduces inhomogeneous broadening of the spectral lines for samples in heterogeneous environments. Another advantage is that the low frequency signals readily pass through conductive materials such as metals due to the increased skin depth; this is not the case for high-field NMR for which the sample containers are usually made of glass, quartz or ceramic.
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.

The physics of magnetic resonance imaging (MRI) concerns fundamental physical considerations of MRI techniques and technological aspects of MRI devices. MRI is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.
Thermoporometry and cryoporometry are methods for measuring porosity and pore-size distributions. A small region of solid melts at a lower temperature than the bulk solid, as given by the Gibbs–Thomson equation. Thus, if a liquid is imbibed into a porous material, and then frozen, the melting temperature will provide information on the pore-size distribution. The detection of the melting can be done by sensing the transient heat flows during phase transitions using differential scanning calorimetry – DSC thermoporometry, measuring the quantity of mobile liquid using nuclear magnetic resonance – NMR cryoporometry (NMRC) or measuring the amplitude of neutron scattering from the imbibed crystalline or liquid phases – ND cryoporometry (NDC).

Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.

A microcoil is a tiny electrical conductor such as a wire in the shape of a spiral or helix which could be a solenoid or a planar structure.
Maurice Goldman is a French physicist and member of the French Academy of Sciences, who is at the origin of developments in the theory of nuclear magnetic resonance.
References
- ↑ Webber, J. Beau W. (2021). "A review of the use of simple time-domain NMR/MRI for material-science". SN Applied Sciences. 3 (10). doi: 10.1007/s42452-021-04791-2 . S2CID 244256779.
- ↑ "Fast Field Cycling Relaxometry". spinscope.com. 2012-04-22. Retrieved 2012-04-22.
- ↑ Davydov V.V.; Velichko E.N.; Karseev A.Yu. (2015). "Nuclear-magnetic mini-relaxometer for liquid and viscous media control". Scientific and Technical Journal of Information Technologies, Mechanics and Optics. 15 (1): 115–121. doi: 10.17586/2226-1494-2015-15-1-115-121 .
- ↑ Кашаев Р.С. (2011). "РАЗВИТИЕ НАУКИ И ОБРАЗОВАНИЯ НА ОСНОВЕ МЕЖДИСЦИПЛИНАРНОГО ПОДХОДА К ПРИМЕНЕНИЮ МЕТОДА ЯДЕРНОГО МАГНИТНОГО РЕЗОНАНСА (ЯМР)". Успехи современного естествознания (2): 82–87.
- ↑ Webber, John Beausire Wyatt; Demin, Pavel (2023). "Digitally Based Precision Time-Domain Spectrometer for NMR Relaxation and NMR Cryoporometry". Micro. 3 (2): 404–433. doi: 10.3390/micro3020028 .