Related Research Articles
Rintatolimod, sold under the tradename Ampligen, is a medication intended for treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). There is some evidence it may improve some ME/CFS symptoms.
The Solve ME/CFS Initiative is an American nonprofit that does research and advocacy for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Long COVID, and other post-infectious diseases. Their stated mission is to assist research into ways to diagnose, treat, or cure these conditions. They also advocate for increased awareness, public funding of research, and access to medical care for patients.

Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) has a long history with an evolution in medical understanding, diagnoses and social perceptions.
Management of ME/CFS focusses on symptoms management, as no treatments that address the root cause of the illness are available. Pacing, or regulating one's activities to avoid triggering worse symptoms, is the most common management strategy for post-exertional malaise. Clinical management varies widely, with many patients receiving combinations of therapies.

Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is an illness with a history of controversy. Although it is classified as an organic disease, it was historically assumed to be psychosocial, and a minority of medical professionals still hold this view. The pathophysiology of ME/CFS remains unclear, there exists many competing diagnostic criteria, and some proposed treatments are controversial. There is a lack of awareness about the condition, which has led to substantiated accusations of patient neglect and harm.

Clinical descriptions of ME/CFS vary. Different groups have produced sets of diagnostic criteria that share many similarities. The biggest differences between criteria are whether post-exertional malaise (PEM) is required, and the number of symptoms needed.
Graded exercise therapy (GET) is a programme of physical activity that starts very slowly and gradually increases over time, intended as a treatment for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Most public health bodies, including the CDC and NICE, consider it ineffective, and its safety is disputed. However, GET still enjoys support among a minority of clinicians and organizations.
David Sheffield Bell is an American physician who has done extensive research on the clinical aspects of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). He has also conducted evaluations and research in pediatric ME/CFS and written numerous articles about the condition.
Daniel Peterson is an American physician in private practice in the state of Nevada, and has been described as a "pioneer" in the treatment of chronic fatigue syndrome (CFS). He graduated from the University of Rochester School of Medicine, Rochester, New York, in 1976 and was an intern and resident at the University of Utah Medical Center from 1976 to 1979. In 1979, he became a diplomate of the American Board of Internal Medicine. He is president of Sierra Internal Medicine of Incline Village, established in 1981.
Dr. José Gilberto Montoya is a prominent researcher known for his contributions to the field of infectious diseases, particularly in the area of chronic fatigue syndrome (CFS) and the role of infectious agents in its development. His research has shed light on the potential involvement of pathogens and immune dysregulation in the pathophysiology of CFS. He was a Professor of Medicine in Infectious Disease at the Stanford University School of Medicine, where he led Stanford's Initiative on Chronic Fatigue Syndrome. He has worked on a wide variety of projects, including research focused on the efficacy of new smallpox vaccines. Additionally, he was the founder and co-director of the Immunocompromised Host Service and works at the Positive Care Clinic at Stanford. He is originally from Cali, Colombia.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a serious long-term illness. People with ME/CFS experience a profound fatigue that does not go away with rest, sleep issues and problems with memory or concentration. They are able to do much less than before they became ill. Further common symptoms include dizziness, nausea and pain. The hallmark symptom is a worsening of the illness hours to days after minor physical or mental activity. This "crash" can last hours to several months.
Rosamund Vallings is a medical doctor, known as one of the leading authorities on Chronic Fatigue Syndrome (ME/CFS) in New Zealand.
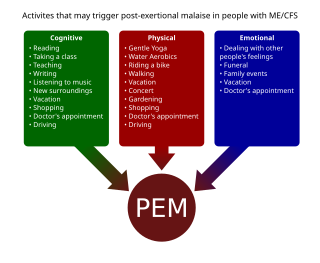
Post-exertional malaise (PEM), sometimes referred to as post-exertional symptom exacerbation (PESE) or post-exertional neuroimmune exhaustion (PENE), is a worsening of symptoms that occurs after minimal exertion. It is the hallmark symptom of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and common in long COVID and fibromyalgia. PEM is often severe enough to be disabling, and is triggered by ordinary activities that healthy people tolerate. Typically, it begins 12–48 hours after the activity that triggers it, and lasts for days, but this is highly variable and may persist much longer. Management of PEM is symptom-based, and patients are recommended to pace their activities to avoid triggering PEM.

Maureen Hanson is an American molecular biologist and Liberty Hyde Bailey Professor in the Department of Molecular Biology and Genetics at Cornell University in Ithaca, New York. She is a joint member of the Section of Plant Biology and Director of the Center for Enervating Neuroimmune Disease. Her research concerns gene expression in chloroplasts and mitochondria, photosynthesis, and the molecular basis of the disease Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).

The Puzzle Solver: A Scientist's Desperate Quest to Cure the Illness that Stole His Son is a book by Tracie White with scientist Ronald W. Davis about Davis's efforts to cure his son Whitney Dafoe, who has very severe myalgic encephalomyelitis, also called chronic fatigue syndrome (ME/CFS). The book was published on January 5, 2021.

DecodeME is an ongoing genome-wide association study searching for genetic risk factors for ME/CFS. With a planned recruitment of 25,000 patients, it is expected to be the largest such study to date. Recruitment closed on 15 November 2023 and results are expected in 2024.
The Open Medicine Foundation (OMF) is a US-based charity that funds research into the illnesses myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), fibromyalgia, post-treatment Lyme disease syndrome, and long COVID.
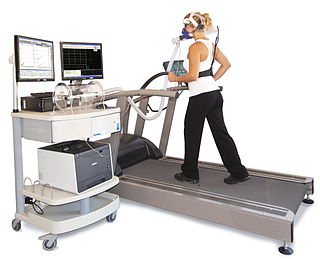
A 2-day CPET is a cardiopulmonary exercise test given on two successive days to measure the effect of post-exertional malaise (PEM) on a patient's ability to exercise. PEM is a cardinal symptom of myalgic encephalomyelitis/chronic fatigue syndrome and is common in long COVID as well.
Andrew Melvin Ramsay (1901–1990) was a British physician, who is known for his research and advocacy on myalgic encephalomyelitis (ME), a chronic disease causing muscle weakness and cognitive dysfunction. Ramsay worked as a consultant at the Royal Free Hospital in London during a mysterious 1955 disease outbreak of what later became known as ME. He studied the disease and similar outbreaks elsewhere. Work by Ramsay showed that although ME seldom caused death, the disease could be highly disabling.
Carmen Scheibenbogen is a German immunologist who is the acting director of the Institute for Medical Immunology of the Charité university hospital in Berlin. She specialises in hematology, oncology and immunology. She leads the Outpatient Clinic for Immunodeficiency and the Fatigue Centre at the Charité hospital. She is one of the few doctors specialised in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in Germany, and also researches long COVID.
References
- 1 2 3 "Happy 75th Birthday to Scientist Ronald W. Davis, PhD". Open Medicine Foundation (video). July 12, 2016. Retrieved May 3, 2019.
- 1 2 "Past Luminary Awards". PMWC 2019 Silicon Valley Luminary & Pioneer Awards. Retrieved May 3, 2019.
- 1 2 "2011 Gruber Genetics Prize: Ronald W. Davis". Gruber Foundation at Yale. 2011. Retrieved May 5, 2019.
- ↑ "Genome Technology Center: History". Stanford Medicine. Palo Alto, California. Retrieved May 3, 2019.
- ↑ "Center Publications – Genome Technology Center". Stanford University School of Medicine. December 12, 2013. Retrieved May 3, 2019.
- 1 2 Allan, Nicole (October 23, 2013). "Who Will Tomorrow's Historians Consider Today's Greatest Inventors?". The Atlantic.
- 1 2 3 Haas, Michaela (January 2, 2021). "A geneticist's biggest challenge: Curing his own son". Al Jazeera. Retrieved June 8, 2021.
- 1 2 3 4 5 6 7 Prior, Ryan (May 13, 2019). "He pioneered technology that fueled the Human Genome Project. Now his greatest challenge is curing his own son". CNN. Retrieved May 14, 2019.
- ↑ "Ronald W. Davis". National Academy of Sciences member directory. Retrieved January 18, 2021.
- ↑ "Ronald W. Davis Honors and Awards". Stanford Medicine Genome Technology Center. May 15, 2017. Retrieved January 18, 2021.
- ↑ University of Pittsburgh University Marketing Communications Webteam. "Ronald W. Davis, PhD – Dickson Prize in Medicine – University of Pittsburgh".
- ↑ Mertz, J. E.; Davis, R. W. (1972). "Cleavage of DNA by RI restriction endonuclease generates cohesive ends". Proceedings of the National Academy of Sciences. 69 (11): 3370–3374. Bibcode:1972PNAS...69.3370M. doi: 10.1073/pnas.69.11.3370 . PMC 389773 . PMID 4343968.
- ↑ Schena, M.; Shalon, D.; Davis, R. W.; Brown, P. O. (1995). "Quantitative monitoring of gene expression patterns with a complementary DNA microarray". Science. 270 (5235): 467–470. Bibcode:1995Sci...270..467S. doi:10.1126/science.270.5235.467. PMID 7569999. S2CID 6720459.
- ↑ Lashkari DA, DeRisi JL, McCusker JH, Namath AF, Gentile C, Hwang SY, Brown PO, Davis RW (1997). "Yeast microarrays for genome wide parallel genetic and gene expression analysis". Proceedings of the National Academy of Sciences. 94 (24): 13057–13062. Bibcode:1997PNAS...9413057L. doi: 10.1073/pnas.94.24.13057 . PMC 24262 . PMID 9371799.
- ↑ Botstein, D.; White, R.; Skolnick, M.; Davis, R. (1980). "Construction of a genetic linkage map in man using restriction fragment length polymorphisms". American Journal of Human Genetics. 32 (3): 314–331. PMC 1686077 . PMID 6247908.
- ↑ "Scientific Advisory Board". Open Medicine Foundation. Agoura Hills, California. 2019. Retrieved May 3, 2018.
- 1 2 "The End ME/CFS Project". Open Medicine Foundation. Retrieved May 3, 2019.
- ↑ "What does the nanoneedle research mean for ME/CFS patients?". Open Medical Foundation (video). April 29, 2019. Retrieved May 4, 2019.
- 1 2 3 4 Chadradhar, Shraddha (April 30, 2019). "An experimental test may help confirm cases of chronic fatigue syndrome". Stat News. Retrieved May 4, 2019.
- ↑ Esfandyarpour, R.; Kashi, A.; Nemat-Gorgani, M.; Wilhelmy, J.; Davis, R. W. (April 29, 2019). "A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)". Proceedings of the National Academy of Sciences. 116 (21): 10250–10257. Bibcode:2019PNAS..11610250E. doi: 10.1073/pnas.1901274116 . PMC 6535016 . PMID 31036648.
- ↑ "Invisible Illness – Stories of Chronic Fatigue Syndrome". Palo Alto Online (video). July 10, 2015. Retrieved May 3, 2019.
- ↑ "Finding an ME/CFS Biomarker, Ronald Davis, Stanford University". ME/CFS Alert 109 (video). September 13, 2019. Retrieved September 20, 2019.