Related Research Articles

The trp operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The trp operon was first characterized in Escherichia coli, and it has since been discovered in many other bacteria. The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed.
The gene rpoF encodes the sigma factor sigma-28, a protein in Escherichia coli and other species of bacteria. Depending on the bacterial species, this gene may be referred to as sigD or fliA. The protein encoded by this gene has been found to be necessary for flagellum formation.
In the field of molecular biology the 6S RNA is a non-coding RNA that was one of the first to be identified and sequenced. What it does in the bacterial cell was unknown until recently. In the early 2000s scientists found out the function of 6S RNA to be as a regulator of sigma 70-dependent gene transcription. All bacterial RNA polymerases have a subunit called a sigma factor. The sigma factors are important because they control how DNA promoter binding and RNA transcription start sites. Sigma 70 was the first one to be discovered in Escherichia coli.

Swarming motility is a rapid and coordinated translocation of a bacterial population across solid or semi-solid surfaces, and is an example of bacterial multicellularity and swarm behaviour. Swarming motility was first reported by Jorgen Henrichsen and has been mostly studied in genus Serratia, Salmonella, Aeromonas, Bacillus, Yersinia, Pseudomonas, Proteus, Vibrio and Escherichia.
In a screen of the Bacillus subtilis genome for genes encoding ncRNAs, Saito et al. focused on 123 intergenic regions (IGRs) over 500 base pairs in length, the authors analyzed expression from these regions. Seven IGRs termed bsrC, bsrD, bsrE, bsrF, bsrG, bsrH and bsrI expressed RNAs smaller than 380 nt. All the small RNAs except BsrD RNA were expressed in transformed Escherichia coli cells harboring a plasmid with PCR-amplified IGRs of B. subtilis, indicating that their own promoters independently express small RNAs. Under non-stressed condition, depletion of the genes for the small RNAs did not affect growth. Although their functions are unknown, gene expression profiles at several time points showed that most of the genes except for bsrD were expressed during the vegetative phase, but undetectable during the stationary phase. Mapping the 5' ends of the 6 small RNAs revealed that the genes for BsrE, BsrF, BsrG, BsrH, and BsrI RNAs are preceded by a recognition site for RNA polymerase sigma factor σA.

Catabolite Control Protein A (CcpA) is a master regulator of carbon metabolism in gram-positive bacteria. It is a member of the LacI/GalR transcription regulator family. In contrast to most LacI/GalR proteins, CcpA is allosterically regulated principally by a protein-protein interaction, rather than a protein-small molecule interaction. CcpA interacts with the phosphorylated form of Hpr and Crh, which is formed when high concentrations of glucose or fructose-1,6-bisphosphate are present in the cell. Interaction of Hpr or Crh modulates the DNA sequence specificity of CcpA, allowing it to bind operator DNA to modulate transcription. Small molecules glucose-6-phosphate and fructose-1,6-bisphosphate are also known allosteric effectors, fine-tuning CcpA function.
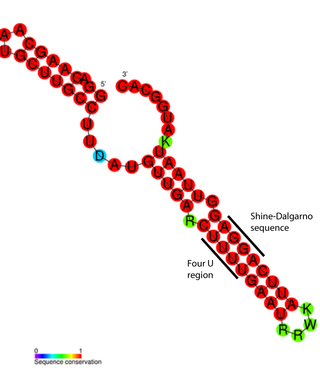
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
Rsa RNAs are non-coding RNAs found in the bacterium Staphylococcus aureus. The shared name comes from their discovery, and does not imply homology. Bioinformatics scans identified the 16 Rsa RNA families named RsaA-K and RsaOA-OG. Others, RsaOH-OX, were found thanks to an RNomic approach. Although the RNAs showed varying expression patterns, many of the newly discovered RNAs were shown to be Hfq-independent and most carried a C-rich motif (UCCC).

Mycobacterium tuberculosis contains at least nine small RNA families in its genome. The small RNA (sRNA) families were identified through RNomics – the direct analysis of RNA molecules isolated from cultures of Mycobacterium tuberculosis. The sRNAs were characterised through RACE mapping and Northern blot experiments. Secondary structures of the sRNAs were predicted using Mfold.
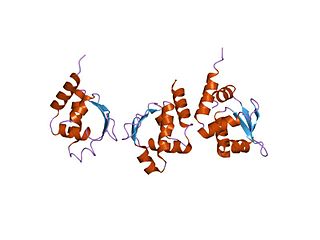
In molecular biology, the CodY protein family consists of several bacterial GTP-sensing transcriptional pleiotropic repressor CodY proteins. CodY has been found to repress the dipeptide transport operon (dpp) of Bacillus subtilis in nutrient-rich conditions. The CodY protein also has a repressor effect on many genes in Lactococcus lactis during growth in milk.

In molecular biology, the KduI/IolB isomerase family is a family of isomerase enzymes that includes 4-deoxy-L-threo-5-hexosulose-uronate ketol-isomerase (KduI) and 5-deoxy-glucuronate isomerase (IolB).
In molecular biology, the PyrG leader is a cis-regulatory RNA element found at the 5' of the PyrG mRNA. The PyrG gene encodes a CTP synthase, which is involved in pyrimidine biosynthesis. The PyrG leader regulates expression of PyrG, PyrG can form into two different hairpin structures, a terminator or an anti-terminator. Under low CTP conditions, guanine (G) residues are incorporated at a specific site within the PyrG leader, these allow base-pairing with a uracil (U)-rich region and the formation of an anti-terminator loop, this results in increased expression of PyrG. Under high CTP conditions the guanines are not added, the anti-terminator loop cannot form and instead a terminator loop is formed, preventing further PyrG expression.
In molecular biology, the FasX small RNA (fibronectin/fibrinogen-binding/haemolytic-activity/streptokinase-regulator-X) is a non-coding small RNA (sRNA) produced by all group A Streptococcus. FasX has also been found in species of group D and group G Streptococcus. FasX regulates expression of secreted virulence factor streptokinase (SKA), encoded by the ska gene. FasX base pairs to the 5' end of the ska mRNA, increasing the stability of the mRNA, resulting in elevated levels of streptokinase expression. FasX negatively regulates the expression of pili and fibronectin-binding proteins on the bacterial cell surface. It binds to the 5' untranslated region of genes in the FCT-region in a serotype-specific manner, reducing the stability of and inhibiting translation of the pilus biosynthesis operon mRNA by occluding the ribosome-binding site through a simple Watson-Crick base-pairing mechanism.
Octanoyl-(GcvH):protein N-octanoyltransferase (EC 2.3.1.204, LIPL, octanoyl-[GcvH]:E2 amidotransferase, YWFL (gene)) is an enzyme with systematic name (glycine cleavage system H)-N6-octanoyl-L-lysine:(lipoyl-carrier protein)-N6-L-lysine octanoyltransferase. This enzyme catalyses the following chemical reaction
The parABS system is a broadly conserved molecular mechanism for plasmid partitioning and chromosome segregation in bacteria. Originally identified as a genetic element required for faithful partitioning of low-copy-number plasmids, it consists of three components: the ParA ATPase, the ParB DNA-binding protein, and the cis-acting parS sequence. The parA and parB genes are typically found in the same operon, with parS elements located within or adjacent to this operon. Collectively, these components function to ensure accurate partitioning of plasmids or whole chromosomes between bacterial daughter cells prior to cell division.
Bacterial small RNAs (sRNA) are an important class of regulatory molecules in bacteria such as Brucella. They are often bound to the chaperone protein Hfq, which allows them to interact with mRNA(s). In Brucella suis 1330 RNA sequencing identified a novel list of 33 sRNAs and 62 Hfq-associated mRNAs. In Brucella melitensis eight novel sRNA genes were identified using bioinformatic and experimental approach. One of them BSR0602 was found to modulate the intracellular survival of B. melitensis. In another large-scale deep sequencing study 1321 sRNAs were identified in B. melitensis. BSR0441 sRNA was further investigated in this study and shown to play role in the intracellular survival. sRNA BM-sr0117 from Brucella melitensis was identified and shown to be bound to and cleaved by Bm-RNase III. AbcR and AbcR2 were studied B. abortus. Seven novel sRNAs were validated and their interaction with a putative target sequence was verified in B. abortus.

Paul Babitzke is a professor of biochemistry and molecular biology and director of the Center for RNA Molecular Biology at Pennsylvania State University.
Transcription-translation coupling is a mechanism of gene expression regulation in which synthesis of an mRNA (transcription) is affected by its concurrent decoding (translation). In prokaryotes, mRNAs are translated while they are transcribed. This allows communication between RNA polymerase, the multisubunit enzyme that catalyzes transcription, and the ribosome, which catalyzes translation. Coupling involves both direct physical interactions between RNA polymerase and the ribosome, as well as ribosome-induced changes to the structure and accessibility of the intervening mRNA that affect transcription.
The Bacteroides thetaiotaomicron genome contains hundreds of small RNAs (sRNAs), discovered through RNA sequencing. These include canonical housekeeping RNA species such as the 6S RNA (SsrS), tmRNA (SsrA), M1 RNA (RnpB) and 4.5S RNA (Ffs) as well as several hundred cis and trans encoded small RNAs. More than 20 candidates have been validated with northern blots and the structures of several members have been characterized through in silico analyses and chemical probing experiments.
References
- ↑ Gimpel M, Preis H, Barth E, Gramzow L, Brantl S (October 13, 2012). "SR1—a small RNA with two remarkably conserved functions". Nucleic Acids Research. 40 (22): 11659–11672. doi:10.1093/nar/gks895. PMC 3526287 . PMID 23034808.
- ↑ Heidrich N, Moll I, Brantl S (2007). "In vitro analysis of the interaction between the small RNA SR1 and its primary target ahrC mRNA". Nucleic Acids Research. 35 (13): 4331–4346. doi:10.1093/nar/gkm439. PMC 1935000 . PMID 17576690.
- ↑ Heidrich N, Chinali A, Gerth U, Brantl S (October 2006). "The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism". Molecular Microbiology. 62 (2): 520–536. doi: 10.1111/j.1365-2958.2006.05384.x . PMID 17020585.
- ↑ North AK, Smith MC, Baumberg S (August 1, 1989). "Nucleotide sequence of a Bacillus subtilis arginine regulatory gene and homology of its product to the Escherichia coli arginine repressor". Gene. 80 (1): 29–38. doi:10.1016/0378-1119(89)90247-3. PMID 2507400.
- ↑ Czaplewski LG, North AK, Smith MC, Baumberg S, Stockley PG (January 1992). "Purification and initial characterization of AhrC: the regulator of arginine metabolism genes in Bacillus subtilis". Mol. Microbiol. 6 (2): 267–275. doi:10.1111/j.1365-2958.1992.tb02008.x. PMID 1312212. S2CID 3219261.
- ↑ Dennis CA, Glykos NM, Parsons MR, Phillips SE (March 2002). "The structure of AhrC, the arginine repressor/activator protein from Bacillus subtilis". Acta Crystallographica Section D. 58 (Pt 3): 421–430. doi:10.1107/s0907444901021692. PMID 11856827.
- ↑ Gimpel M, Heidrich N, Mäder U, Krügel H, Brantl S (May 2010). "A dual-function sRNA from B. subtilis: SR1 acts as a peptide encoding mRNA on the gapA operon" (PDF). Molecular Microbiology. 76 (4): 990–1009. doi: 10.1111/j.1365-2958.2010.07158.x . PMID 20444087.
- ↑ Licht A, Preis S, Brantl S (October 2005). "Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in Bacillus subtilis". Molecular Microbiology. 58 (1): 189–206. doi:10.1111/j.1365-2958.2005.04810.x. PMID 16164558. S2CID 29961832.
- ↑ Licht A, Brantl S (October 2009). "The transcriptional repressor CcpN from Bacillus subtilis uses different repression mechanisms at different promoters". Journal of Biological Chemistry. 284 (44): 30032–30038. doi: 10.1074/jbc.M109.033076 . PMC 2781557 . PMID 19726675.