
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.
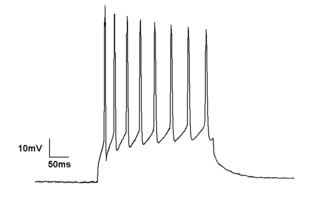
Electrophysiology is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring.
In the field of electronic devices, roll-to-roll processing, also known as web processing, reel-to-reel processing or R2R, is the process of creating electronic devices on a roll of flexible plastic, metal foil, or flexible glass. In other fields predating this use, it can refer to any process of applying coating, printing, or performing other processes starting with a roll of a flexible material and re-reeling after the process to create an output roll. These processes, and others such as sheeting, can be grouped together under the general term converting. When the rolls of material have been coated, laminated or printed they can be subsequently slit to their finished size on a slitter rewinder.

A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.
Nanosensors are nanoscale devices that measure physical quantities and convert these to signals that can be detected and analyzed. There are several ways proposed today to make nanosensors; these include top-down lithography, bottom-up assembly, and molecular self-assembly. There are different types of nanosensors in the market and in development for various applications, most notably in defense, environmental, and healthcare industries. These sensors share the same basic workflow: a selective binding of an analyte, signal generation from the interaction of the nanosensor with the bio-element, and processing of the signal into useful metrics.

Surface-enhanced Raman spectroscopy or surface-enhanced Raman scattering (SERS) is a surface-sensitive technique that enhances Raman scattering by molecules adsorbed on rough metal surfaces or by nanostructures such as plasmonic-magnetic silica nanotubes. The enhancement factor can be as much as 1010 to 1011, which means the technique may detect single molecules.

Printed electronics is a set of printing methods used to create electrical devices on various substrates. Printing typically uses common printing equipment suitable for defining patterns on material, such as screen printing, flexography, gravure, offset lithography, and inkjet. By electronic-industry standards, these are low-cost processes. Electrically functional electronic or optical inks are deposited on the substrate, creating active or passive devices, such as thin film transistors; capacitors; coils; resistors. Some researchers expect printed electronics to facilitate widespread, very low-cost, low-performance electronics for applications such as flexible displays, smart labels, decorative and animated posters, and active clothing that do not require high performance.
A conductive textile is a fabric which can conduct electricity. Conductive textiles known as lamé are made with guipé thread or yarn that is conductive because it is composed of metallic fibers wrapped around a non-metallic core or has a metallic coating. A different way of achieving conductivity is to weave metallic strands into the textile.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.
Amperometry in chemistry is detection of ions in a solution based on electric current or changes in electric current.
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.
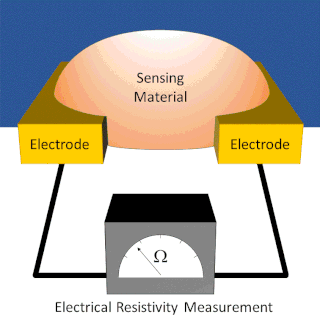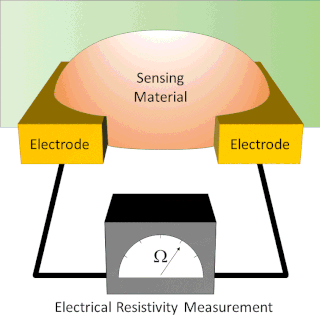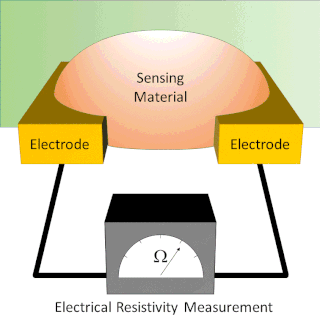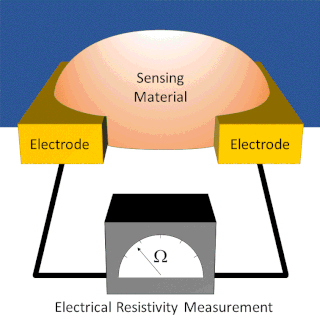
A chemiresistor is a material that changes its electrical resistance in response to changes in the nearby chemical environment. Chemiresistors are a class of chemical sensors that rely on the direct chemical interaction between the sensing material and the analyte. The sensing material and the analyte can interact by covalent bonding, hydrogen bonding, or molecular recognition. Several different materials have chemiresistor properties: metal-oxide semiconductors, some conductive polymers, and nanomaterials like graphene, carbon nanotubes and nanoparticles. Typically these materials are used as partially selective sensors in devices like electronic tongues or electronic noses.

A field-effect transistor-based biosensor, also known as a biosensor field-effect transistor, field-effect biosensor (FEB), or biosensor MOSFET, is a field-effect transistor that is gated by changes in the surface potential induced by the binding of molecules. When charged molecules, such as biomolecules, bind to the FET gate, which is usually a dielectric material, they can change the charge distribution of the underlying semiconductor material resulting in a change in conductance of the FET channel. A Bio-FET consists of two main compartments: one is the biological recognition element and the other is the field-effect transistor. The BioFET structure is largely based on the ion-sensitive field-effect transistor (ISFET), a type of metal–oxide–semiconductor field-effect transistor (MOSFET) where the metal gate is replaced by an ion-sensitive membrane, electrolyte solution, and reference electrode.

Electrochemical stripping analysis is a set of analytical chemistry methods based on voltammetry or potentiometry that are used for quantitative determination of ions in solution. Stripping voltammetry have been employed for analysis of organic molecules as well as metal ions. Carbon paste, glassy carbon paste, and glassy carbon electrodes when modified are termed as chemically modified electrodes and have been employed for the analysis of organic and inorganic compounds.
Paper-based microfluidics are microfluidic devices that consist of a series of hydrophilic cellulose or nitrocellulose fibers that transport fluid from an inlet through the porous medium to a desired outlet or region of the device, by means of capillary action. This technology builds on the conventional lateral flow test which is capable of detecting many infectious agents and chemical contaminants. The main advantage of this is that it is largely a passively controlled device unlike more complex microfluidic devices. Development of paper-based microfluidic devices began in the early 21st century to meet a need for inexpensive and portable medical diagnostic systems.

Aptamers, single-stranded RNA and DNA sequences, bind to an analyte and change their conformation. They function as nucleic acids selectively binding molecules such as proteins, bacteria cells, metal ions, etc. Aptamers can be developed to have precise specificity to bind to a desired target. Aptamers change conformation upon binding, altering the electrochemical properties which can be measured. The Systematic Evolution of Ligands by Exponential Enrichment (SELEX) process generates aptamers. Electrochemical aptamer-based (E-AB) biosensors is a device that takes advantage of the electrochemical and biological properties of aptamers to take real time, in vivo measurements.
Raman spectroelectrochemistry (Raman-SEC) is a technique that studies the inelastic scattering or Raman scattering of monochromatic light related to chemical compounds involved in an electrode process. This technique provides information about vibrational energy transitions of molecules, using a monochromatic light source, usually from a laser that belongs to the UV, Vis or NIR region. Raman spectroelectrochemistry provides specific information about structural changes, composition and orientation of the molecules on the electrode surface involved in an electrochemical reaction, being the Raman spectra registered a real fingerprint of the compounds.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.
A chemical sensor array is a sensor architecture with multiple sensor components that create a pattern for analyte detection from the additive responses of individual sensor components. There exist several types of chemical sensor arrays including electronic, optical, acoustic wave, and potentiometric devices. These chemical sensor arrays can employ multiple sensor types that are cross-reactive or tuned to sense specific analytes.












