
Disease surveillance is an epidemiological practice by which the spread of disease is monitored in order to establish patterns of progression. The main role of disease surveillance is to predict, observe, and minimize the harm caused by outbreak, epidemic, and pandemic situations, as well as increase knowledge about which factors contribute to such circumstances. A key part of modern disease surveillance is the practice of disease case reporting.

GISAID is a global science initiative and primary source established in 2008 that provides open-access to genomic data of influenza viruses and the coronavirus responsible for the COVID-19 pandemic. On January 10, 2020, the first whole-genome sequences of SARS-CoV-2 were made available on GISAID, which enabled global responses to the pandemic, including the development of the first vaccines and diagnostic tests to detect SARS-CoV-2. The database has become the world's largest repository for SARS-CoV-2 sequences. GISAID facilitates genomic epidemiology and real-time surveillance to monitor the emergence of new COVID-19 viral strains across the planet.

Influenza-like illness (ILI), also known as flu-like syndrome or flu-like symptoms, is a medical diagnosis of possible influenza or other illness causing a set of common symptoms. These include fever, shivering, chills, malaise, dry cough, loss of appetite, body aches, and nausea, typically in connection with a sudden onset of illness. In most cases, the symptoms are caused by cytokines released by immune system activation, and are thus relatively non-specific.
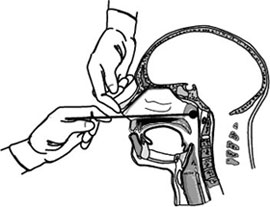
A nasopharyngeal swab is a device used for collecting a sample of nasal secretions from the back of the nose and throat. The sample is then analyzed for the presence of organisms or other clinical markers for disease. This diagnostic method is commonly used in suspected cases of whooping cough, diphtheria, influenza, and various types of diseases caused by the coronavirus family of viruses, including SARS, MERS, and COVID-19.

The COVID-19 pandemic, also known as the coronavirus pandemic, is an ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel virus was first identified from an outbreak in the Chinese city of Wuhan in December 2019, and attempts to contain it there failed, allowing it to spread across the globe. The World Health Organization (WHO) declared a Public Health Emergency of International Concern on 30 January 2020 and a pandemic on 11 March 2020. As of 26 January 2022, the pandemic had caused more than 360 million cases and 5.62 million deaths, making it one of the deadliest in history.

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was identified in Wuhan, China, in December 2019. The disease has since spread worldwide, leading to an ongoing pandemic.

COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main branches detect either the presence of the virus or of antibodies produced in response to infection. Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests instead show whether someone once had the disease. They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection. It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.
Allison McGeer is a Canadian infectious disease specialist in the Sinai Health System, and a Professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto. She also appointed at the Dalla Lana School of Public Health and a Senior Clinician Scientist at the Lunenfeld-Tanenbaum Research Institute. McGeer has led investigations into the severe acute respiratory syndrome outbreak in Toronto and worked alongside Donald Low. During the COVID-19 pandemic, McGeer has studied how SARS-CoV-2 survives in the air.

This article documents the chronology and epidemiology of SARS-CoV-2 in 2019, the virus that causes coronavirus disease 2019 (COVID-19) and is responsible for the COVID-19 pandemic. The first human cases of COVID-19 known to have been identified were in Wuhan, Hubei, China, in December 2019.
Helen Y. Chu is an American immunologist who is an Assistant Professor of Medicine at the University of Washington. Her research considers maternal immunization, with a focus on influenza and respiratory syncytial virus. During the COVID-19 pandemic, Chu was the first physician to recognise community transmission of the coronavirus disease within the United States.

A coronavirus breathalyzer is a diagnostic medical device enabling the user to test with 90% or greater accuracy the presence of severe acute respiratory syndrome coronavirus 2 in an exhaled breath. As of the first half of 2020, the idea of a practical coronavirus breathalyzer was concomitantly developed by unrelated research groups in Australia, Canada, Finland, Germany, Indonesia, Israel, Netherlands, Poland, Singapore, United Kingdom and USA.

The glossary of the COVID-19 pandemic is a list of definitions of terms relating to the COVID-19 pandemic. The pandemic has created and popularized many terms relating to disease and videoconferencing.

The COVID-19 Genomics UK Consortium (COG-UK) is a group of public health agencies and academic institutions in the United Kingdom created in April 2020 to collect, sequence and analyse genomes of SARS-CoV-2 as part of COVID-19 pandemic response. The consortium comprises the UK's four public health agencies, National Health Service organisations, academic partners and the Wellcome Sanger Institute. The consortium is known for first identifying the SARS-CoV-2 Alpha variant in November 2020. As of January 2021, 45% of all SARS-CoV-2 sequences uploaded to the GISAID sequencing database originated from COG-UK.
Curative, Inc. is a healthcare startup company best known for scaling COVID-19 testing and COVID-19 vaccinations during the COVID-19 pandemic. Headquartered in San Dimas, California, and operating throughout the United States, the company was founded in January 2020 by Fred Turner, Isaac Turner, and Vlad Slepnev to create new diagnostic tests for sepsis and to improve outcomes for sepsis patients. In response to an urgent, unmet need for COVID-19 test development and production in the United States, Curative rapidly shifted focus in March 2020. The company's research team developed a new test for SARS-CoV-2 that utilized oral swabs rather than nasopharyngeal swabs. The Curative test was designed with a scalable process and opportunities to reduce healthcare worker exposure risk and the amount of personal protective equipment (PPE) used. An independent manufacturing and supply chain model was adopted to avoid competing with existing COVID-19 test companies for limited supplies and laboratory capacity.

The development of COVID-19 tests was a major public health priority during the early months of the COVID-19 pandemic.

COVID-19 rapid antigen tests or RATs, also frequently called COVID-19 lateral flow tests or LFTs, are rapid antigen tests used to detect SARS-COV-2 infection (COVID-19). They are quick to implement with minimal training, and offer significant cost advantages, costing a fraction of other forms of COVID-19 testing and give users a result within 5–30 minutes. Rapid antigen tests are used in several countries as part of mass testing or population-wide screening approaches. They are thought to be valuable for identifying individuals who are asymptomatic and could potentially spread the virus to other people, who would otherwise not know they were infected. This differs from other forms of COVID-19 testing, such as polymerase chain reaction (PCR), that are generally seen to be a useful test for symptomatic individuals, as they have a higher sensitivity and can more accurately identify cases.

Anne Louise Wyllie is a New Zealand microbiologist who was the lead author of a 2020 research article which led to the development of the SalivaDirect PCR method of testing saliva for SARS-CoV-2, the virus that causes COVID-19. She has also worked on community studies to better understand pneumococcal disease. She is a research scientist in epidemiology with the Public Health Modeling Unit at Yale University.

The United Kingdom's response to the COVID-19 pandemic with consists of various measures by the national health services community; the British and devolved governments; the military; and the research sector.
The New Zealand Microbiology Network (NZMN) is an advisory group to the Ministry of Health in New Zealand. It was established in 2014 through a contract from the Ministry of Health to the Institute of Environmental Science and Research (ESR).










