Related Research Articles

Astatine is a chemical element with the symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. A sample of the pure element has never been assembled, because any macroscopic specimen would be immediately vaporized by the heat of its radioactivity.
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.
A chemical element is a chemical substance that cannot be broken down into other substances. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. This is in contrast to chemical compounds and mixtures, which contain atoms with more than one atomic number.

Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223, has a half-life of only 22 minutes. It is the second-most electropositive element, behind only caesium, and is the second rarest naturally occurring element. Francium's isotopes decay quickly into astatine, radium, and radon. The electronic structure of a francium atom is [Rn] 7s1; thus, the element is classed as an alkali metal.

Glenn Theodore Seaborg was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work in this area also led to his development of the actinide concept and the arrangement of the actinide series in the periodic table of the elements.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group (XVII) or group (VII).

Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All known synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years.
The transuranium elements are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. With the exception of neptunium and plutonium, all do not occur naturally on Earth and are synthetic.

Otto Hahn was a German chemist who was a pioneer in the fields of radioactivity and radiochemistry. He is referred to as the father of nuclear chemistry and father of nuclear fission. Hahn and Lise Meitner discovered radioactive isotopes of radium, thorium, protactinium and uranium. He also discovered the phenomena of atomic recoil and nuclear isomerism, and pioneered rubidium–strontium dating. In 1938, Hahn, Lise Meitner and Fritz Strassmann discovered nuclear fission, for which Hahn received the 1944 Nobel Prize for Chemistry. Nuclear fission was the basis for nuclear reactors and nuclear weapons.

Edwin Mattison McMillan was an American physicist credited with being the first-ever to produce a transuranium element, neptunium. For this, he shared the 1951 Nobel Prize in Chemistry with Glenn Seaborg.

Luis Walter Alvarez was an American experimental physicist, inventor, and professor who was awarded the Nobel Prize in Physics in 1968 for his discovery of resonance states in particle physics using the hydrogen bubble chamber. In 2007 the American Journal of Physics commented, "Luis Alvarez was one of the most brilliant and productive experimental physicists of the twentieth century."
The year 1940 in science and technology involved some significant events, listed below.

Emilio Gino Segrè was an Italian and later naturalized American physicist and Nobel laureate, who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was awarded the Nobel Prize in Physics in 1959 along with Owen Chamberlain.
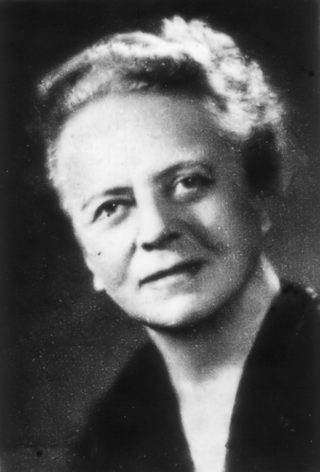
Ida Noddack, néeTacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg, she discovered element 75, rhenium. She was nominated three times for the Nobel Prize in Chemistry.

The MAUD Committee was a British scientific working group formed during the Second World War. It was established to perform the research required to determine if an atomic bomb was feasible. The name MAUD came from a strange line in a telegram from Danish physicist Niels Bohr referring to his housekeeper, Maud Ray.

Horia Hulubei was a Romanian nuclear physicist, known for his contributions to the development of X-ray spectroscopy.

Yvette Cauchois was a French physicist known for her contributions to x-ray spectroscopy and x-ray optics, and for pioneering European synchrotron research.

Nuclear fission was discovered in December 1938 by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Fission is a nuclear reaction or radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei and often other particles. The fission process often produces gamma rays and releases a very large amount of energy, even by the energetic standards of radioactive decay. Scientists already knew about alpha decay and beta decay, but fission assumed great importance because the discovery that a nuclear chain reaction was possible led to the development of nuclear power and nuclear weapons. Hahn was awarded the 1944 Nobel Prize in Chemistry for the discovery of nuclear fission.
References
- ↑ Fontani, Marco (6–10 September 2005). The Twilight of the Naturally-Occurring Elements: Moldavium (Ml), Sequanium (Sq) and Dor (Do). Fifth International Conference on the History of Chemistry. Lisbon. Archived from the original (DOC) on 2006-02-24.
- ↑ Hulubei, H.; Cauchois, Y. (1939). "Nouvelles recherches sur l'élément 93 naturel". Comptes rendus. 209: 476–479.
- ↑ Mcmillan, Edwin; Abelson, Philip (1940). "Radioactive Element 93". Physical Review. 57 (12): 1185. Bibcode:1940PhRv...57.1185M. doi: 10.1103/PhysRev.57.1185.2 .