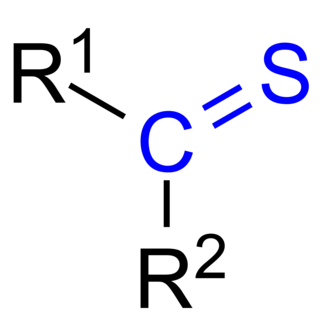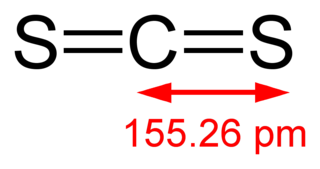
Carbon disulfide is an inorganic compound with the chemical formula CS2 and structure S=C=S. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis. Pure carbon disulfide has a pleasant, ether- or chloroform-like odor, but commercial samples are usually yellowish and are typically contaminated with foul-smelling impurities.
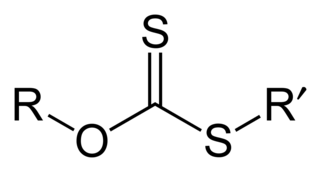
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
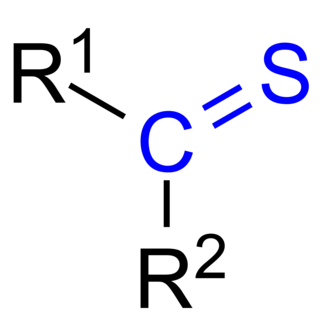
In organic chemistry, thioketones are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Thus the simplest thioketone is thioacetone, the sulfur analog of acetone. Unhindered alkylthioketones typically tend to form polymers or rings.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.

Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−. Related salts are also known including Na2PtCl4, which is brown-colored and soluble in alcohols, and quaternary ammonium salts, which are soluble in a broader range of organic solvents.
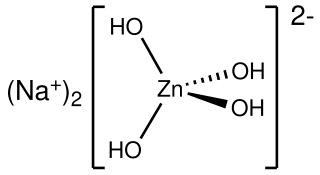
Sodium zincate refers to anionic zinc oxides or hydroxides, depending on conditions. In the applications of these materials, the exact formula is not necessarily important and it is likely that aqueous zincate solutions consist of mixtures.
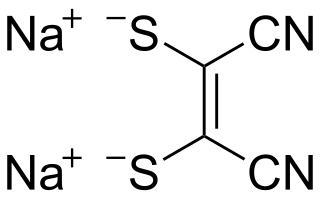
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C2(CN)2. The name refers to the cis compound, structurally related to maleonitrile. Maleonitriledithiolate is often abbreviated mnt. It is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate. It is a prototypical non-innocent ligand in coordination chemistry. Several complexes are known, such as [Ni(mnt)2]2−.
Phenylphosphine is an organophosphorus compound with the chemical formula C6H5PH2. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry.
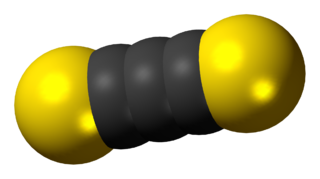
Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.
Thiocarbonate describes a family of anions with the general chemical formula CS
3−xO2−
x (x = 0, 1, or 2):
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.
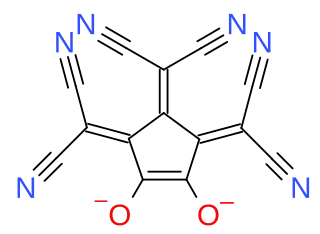
Croconate blue or 1,2,3-tris(dicyanomethylene)croconate is a divalent anion with chemical formula C
14N
6O2−
2 or ((N≡C−)2C=)3(C5O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of three oxygen atoms by dicyanomethylene groups =C(−C≡N)2. The term Croconate Blue as a dye name specifically refers to the dipotassium salt K
2C
14N
6O
2.
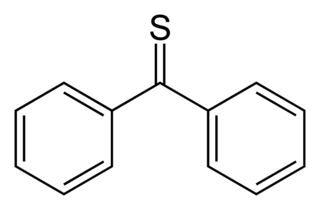
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.
In organic chemistry, thiocarboxylic acids or carbothioic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form and a thiol form. These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.

2-Mercaptobenzothiazole is an organosulfur compound with the formula C6H4(NH)SC=S. A white solid, it is used in the sulfur vulcanization of rubber.
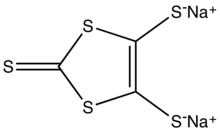
In organosulfur chemistry, 1,3-dithioles are a class of heterocycles based on the parent compound 1,3-dithiacyclopentene (also known as 1,3-dithiole). The ligand dmit2- is a 1,3-dithiole. Heating solutions of Na2dmit gives the isomeric disulfide, a 1,2-dithiole.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.
![Structure of [Zn(dmit)2] . DOQXOW.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/28/DOQXOW.png/220px-DOQXOW.png)